Home > Press > Dancing in the dark: how proteins and salts interact
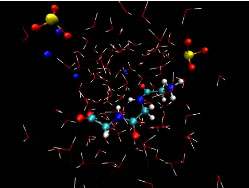 |
| Simulation of the interaction between triglycine and dissolved sodium sulfite in water shows the long chain-like triglycine molecule (center) interacting directly with sulfite anions (tripods of yellow and red atoms) while also interacting via multiple hydrogen bonds (thin red or blue lines) with the surrounding water molecules (red and white sticks). Courtesy Berkeley Lab |
Abstract:
Scientists are getting a new look at how proteins interact with simple salts in water, and what impacts these interactions may have on protein structures at the atomic level.
Dancing in the dark: how proteins and salts interact
Berkeley, CA | Posted on August 13th, 2010To study nanostructures in real environments, Berkeley Lab scientists have combined theoretical and experimental approaches to glimpse into a protein's interaction with simple salts in water. Enabled by x-ray absorption simulation software developed at Berkeley Lab's Molecular Foundry, these findings shed new light on how salts impact protein structure at the atomic level.
Traditional crystallographic techniques, such as x-ray diffraction, provide a profile of ordered materials with static structures. However, for dynamic or complex systems in which the atomic structure is rapidly changing, more sophisticated methods are needed. Now, Berkeley Lab scientists have applied x-ray absorption spectroscopy to study a model protein, triglycine—a short chain of three molecules of the simplest amino acid, glycine. By simulating this molecule's x-ray absorption spectrum the team has show how its chain kinks and straightens in response to ions in solution.
"Watching a molecule in solution is like watching a marionette—you can see it bending in response to making and breaking of hydrogen bonds," said David Prendergast, a staff scientist in the Theory of Nanostructures Facility at the Molecular Foundry. "A concrete knowledge of how ions influence this behavior comes from using molecular dynamics simulations, which show persistent differences in structure on nanosecond timescales. From this data we can generate x-ray absorption spectra which can then be compared with experimental results."
In a specialized x-ray absorption experiment called near edge x-ray absorption fine structure (NEXAFS), x-rays are used to probe the chemical bonding and environment of specific elements in a molecule or nanostructure, such as the nitrogen atoms in a triglycine molecule. Coupled with a liquid microjet technology developed at Berkeley Labs, NEXAFS has been previously used to examine how proteins dissolve and crystallize in the presence of various ions .
Prendergast's software can now simulate NEXAFS data by averaging a series of snapshots taken from a molecular dynamics simulation of a given molecule. This software is a critical tool for interpreting NEXAFS data from complex, dynamic systems, as the probe times in these measurements are too slow—seconds rather than nanoseconds—to reveal structural differences at the nanoscale.
"Previous studies from our group have shown the development of x-ray absorption spectroscopy of liquid microjets provides a new atom-sensitive probe of the interactions between aqueous ions, but it is the advent of this new theory that provides the first reliable molecular-level interpretation of these data," said Richard Saykally, a Berkeley Lab chemist and professor of chemistry at the University of California at Berkeley. "Here we see this new combination of theory and experiment applied to one of the most important problems in biophysical chemistry."
Prendergast says his molecular dynamics technique can be used to model x-ray spectra of a biological system with known structure to determine its local interactions, what causes it to form a particular structure, and why it takes on a particular conformation—all by simulating the spectra of a series of individual snapshots and comparing with experimental results. These simulations are computationally intensive and rely heavily on the large-scale supercomputing infrastructure provided by Berkeley Lab's National Energy Research Scientific Computing Center (NERSC).
"Although these effects are a fundamental part of nature, they are still poorly understood," said Craig Schwartz, a researcher working with Prendergast and Saykally, whose graduate work led to this publication. "The experimental sensitivity of NEXAFS, coupled with a breakthrough in theory, gave us new insight into how these molecules interact."
The researchers anticipate demand from other groups exploring water (or other solvent) interactions, as well as both soft materials (such as polymers) and inorganic materials (oxides and metal surfaces) that are directly relevant to energy-related applications in catalysis, battery technology and photovoltaics. In addition, as x-ray free electron laser sources become available to scientists, a richer experimental data set will be available to augment theoretical findings.
A paper reporting this research titled, "Investigation of protein conformation and interactions with salts via X-ray absorption spectroscopy," appears in Proceedings of the National Academy of Sciences and is available to subscribers online (*). Co-authoring the paper with Schwartz, Prendergast and Saykally were Janel Uejio, Andrew Duffin, Alice England and Daniel Kelly.
This work at the Molecular Foundry and Advanced Light Source was supported by DOE's Office of Science. Computational resources were provided by NERSC, a DOE advanced scientific computing research user facility.
(*) www.pnas.org/content/107/32/14008.abstract
####
About Berkeley Lab
Berkeley Lab is a U.S. Department of Energy national laboratory located in Berkeley, California. It conducts unclassified scientific research and is managed by the University of California.
For more information, please click here
Contacts:
Aditi Risbud (510)486-4861
Copyright © Berkeley Lab
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Physics
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Videos/Movies
![]() New X-ray imaging technique to study the transient phases of quantum materials December 29th, 2022
New X-ray imaging technique to study the transient phases of quantum materials December 29th, 2022
![]() Solvent study solves solar cell durability puzzle: Rice-led project could make perovskite cells ready for prime time September 23rd, 2022
Solvent study solves solar cell durability puzzle: Rice-led project could make perovskite cells ready for prime time September 23rd, 2022
![]() Scientists prepare for the world’s smallest race: Nanocar Race II March 18th, 2022
Scientists prepare for the world’s smallest race: Nanocar Race II March 18th, 2022
![]() Visualizing the invisible: New fluorescent DNA label reveals nanoscopic cancer features March 4th, 2022
Visualizing the invisible: New fluorescent DNA label reveals nanoscopic cancer features March 4th, 2022
Software
![]() Visualizing nanoscale structures in real time: Open-source software enables researchers to see materials in 3D while they're still on the electron microscope August 19th, 2022
Visualizing nanoscale structures in real time: Open-source software enables researchers to see materials in 3D while they're still on the electron microscope August 19th, 2022
![]() Luisier wins SNSF Advanced Grant to develop simulation tools for nanoscale devices July 8th, 2022
Luisier wins SNSF Advanced Grant to develop simulation tools for nanoscale devices July 8th, 2022
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Academic/Education
![]() Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
![]() Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Tools
![]() Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








