Home > Press > New Tissue-Hugging Implant Maps Heart Electrical Activity in Unprecedented Detail
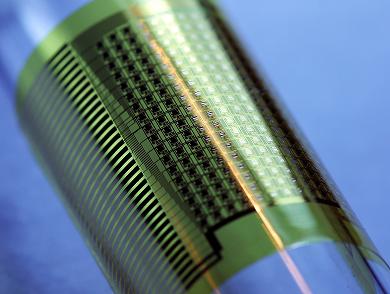 |
| A new type of implantable device that uses flexible silicon technology. Credit: Dae-Hyeong Kim, PhD, University of Illinois. |
Abstract:
A team of cardiologists, materials scientists, and bioengineers have created and tested a new type of implantable device for measuring the heart's electrical output that they say is a vast improvement over current devices. The new device represents the first use of flexible silicon technology for a medical application.
New Tissue-Hugging Implant Maps Heart Electrical Activity in Unprecedented Detail
Philadelphia, PA | Posted on March 28th, 2010"We believe that this technology may herald a new generation of active, flexible, implantable devices for applications in many areas of the body," says co-senior author Brian Litt, an associate professor of Neurology at the University of Pennsylvania School of Medicine and also an associate professor of Bioengineering in Penn's School of Engineering and Applied Science. "Initially, we plan to apply our findings to the design of devices for localizing and treating abnormal heart rhythms. We believe these new devices will allow doctors to more quickly, safely, and accurately target and destroy abnormal areas of the heart that are responsible for life-threatening cardiac arrhythmias.
"Implantable silicon-based devices have the potential to serve as tools for mapping and treating epileptic seizures, providing more precise control over deep brain stimulation, as well as other neurological applications," says Story Landis, PhD, director of the National Institute of Neurological Disorders and Stroke, which provided support for the study. "We are excited by the proof of concept evident in the investigators' ability to map cardiac activity in a large animal model."
"The new devices bring electronic circuits right to the tissue, rather than having them located remotely, inside a sealed can that is placed elsewhere in the body, such as under the collar bone or in the abdomen," explains Litt. "This enables the devices to process signals right at the tissues, which allows them to have a much higher number of electrodes for sensing or stimulation than is currently possible in medical devices."
Now, for example, devices for mapping and eliminating life-threatening heart rhythms allow for up to 10 wires in a catheter that is moved in and around the heart, and is connected to rigid silicon circuits distant from the target tissue. This design limits the complexity and resolution of devices since the electronics cannot get wet or touch the target tissue.
The team describes their proof-of-principle findings in the cover article of this week's Science Translational Medicine.
The team tested the new devices - made of nanoscale, flexible ribbons of silicon embedded with 288 electrodes, forming a lattice-like array of hundreds of connections - on the heart of a porcine animal model. The tissue-hugging shape allows for measuring electrical activity with greater resolution in time and space. The new device can also operate when immersed in the body's salty fluids. The devices can collect large amounts of data from the body, at high speed. This allowed the researchers to map electrical activity on the heart of the large animal.
"Our hope is to use this technology for many other kinds of medical applications, for example to treat brain diseases like epilepsy and movement disorders," adds Litt and co-senior author John Rogers, PhD, from the University of Illinois.
In this experiment, the researchers built a device to map waves of electrical activity in the heart of a large animal. The device uses the 288 contacts and more than 2,000 transistors spaced closely together, while standard clinical systems usually use about five to 10 contacts and no active transistors. "We demonstrated high-density maps of electrical activity on the heart recorded from the device, during both natural and paced beats," says co-author David Callans, professor of medicine at Penn.
"We also plan to design advanced, ‘intelligent' pacemakers that can improve the pumping function of hearts weakened by heart attacks and other diseases." For each of these applications, the team is conducting experiments to test flexible devices in animals before starting human trials.
Another focus of ongoing work is to develop similar types of devices that are not only flexible, like a sheet of plastic, but fully stretchable, like a rubber band. The ability to fully conform and wrap around large areas of curved tissues will require stretchability, as well as flexibility. "The next big step in this new generation of implantable devices will be to find a way to move the power source onto them," says Rogers. "We're still working on a solution to that problem."
This research is a result of a collaboration between the Rogers laboratory, where the flexible electronics technology in the devices was developed and fabricated, and Litt's bioengineering laboratory at Penn, where the medical applications were designed and tested. Heart rhythm experiments were designed and performed in Callans' cardiology laboratory. Mechanical engineers Younggang Huang, PhD, and Jianliang Xiao at Northwestern University and University of Illinois performed the mechanical modeling and design that enables the devices to wrap around the heart and other irregular, curved organs. Litt and Rogers note that the core of their collaboration is Penn Bioengineering PhD student Jonathan Viventi and University of Illinois post-doctoral fellow Dae-Hyeong Kim, PhD, who are co-first authors on the publication. The work was also supported by Joshua Moss, a cardiology fellow at Penn, and several undergraduates and master's students.
The research was funded by National Institute of Neurological Disorders and Stroke, the Klingenstein Foundation, the Epilepsy Therapy Project, and the University of Pennsylvania Schools of Engineering and Medicine.
####
For more information, please click here
Contacts:
Karen Kreeger
215-349-5658
Copyright © University of Pennsylvania
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Academic/Education
![]() Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
![]() Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Nanomedicine
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
![]() Researchers develop artificial building blocks of life March 8th, 2024
Researchers develop artificial building blocks of life March 8th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








