Home > Press > Seeing the Quantum in Chemistry: JILA Scientists Control Chemical Reactions of Ultracold Molecules
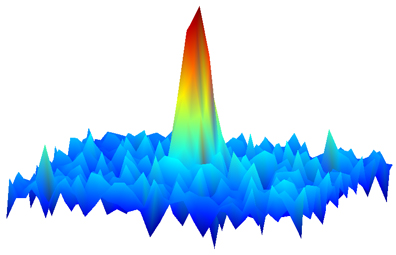 |
| One of the first-ever images of a molecular gas in which each molecule is in its lowest possible energy state. The gas has just been released from a trap created by lasers. The molecules are near absolute zero, a temperature at which quantum properties reign. The image – made by detecting the absorption of laser light by the molecules -- reveals their spatial distribution, with density indicated by peak height and false color. The fact that such an image can be created indicates the molecular quantum gas is dense enough to enable scientists to observe novel interactions among the molecules. Credit: D. Wang/JILA |
Abstract:
Physicists at JILA have for the first time observed chemical reactions near absolute zero, demonstrating that chemistry is possible at ultralow temperatures and that reaction rates can be controlled using quantum mechanics, the peculiar rules of submicroscopic physics.
Seeing the Quantum in Chemistry: JILA Scientists Control Chemical Reactions of Ultracold Molecules
Gaithersburg, MD | Posted on February 11th, 2010The new results and techniques, described in the Feb. 12 issue of Science,* will help scientists understand previously unknown aspects of how molecules interact, a key to advancing biology, creating new materials, producing energy and other research areas. The new JILA work also will aid studies of quantum gases (in which particles behave like waves) and exotic physics spanning the quantum and macroscopic worlds. It may provide practical tools for "designer chemistry" and other applications such as precision measurements and quantum computing.
JILA is a joint institute of the National Institute of Standards and Technology (NIST) and the University of Colorado at Boulder. A NIST theorist at the Joint Quantum Institute, a collaborative venture of NIST and the University of Maryland, also contributed to the research.
"It's perfectly reasonable to expect that when you go to the ultracold regime there would be no chemistry to speak of," says NIST physicist Deborah Jin, leader of one JILA group involved in the experiments. "This paper says no, there's a lot of chemistry going on."
"We are observing a new fundamental aspect of chemistry - it gives us a new ‘knob' to understand and control reactions," adds NIST physicist Jun Ye, leader of the second JILA group involved in the research.
The Science paper is a follow-up to the same research team's 2008 report of the first high-density gas of stable, strongly interacting ultracold molecules, each consisting of two different atoms bonded together (see www.nist.gov/public_affairs/releases/ultracold_polar_molecules.html).
Ultracold molecules are a hot research area because they may offer more diverse insights and applications than ultracold atoms, which scientists have deftly manipulated for more than 20 years.
Scientists have long known how to control the internal states of molecules, such as their rotational and vibrational energy levels. In addition, the field of quantum chemistry has existed for decades to study the effects of the quantum behavior of electrons and nuclei—constituents of molecules. But until now scientists have been unable to observe direct consequences of quantum mechanical motions of whole molecules on the chemical reaction process. Creating simple molecules and chilling them almost to a standstill makes this possible by presenting a simpler and more placid environment that can reveal subtle, previously unobserved chemical phenomena.
By precisely controlling the ultracold molecules' internal states—electronic energy levels, vibrations, rotations and nuclear spin (or angular momentum)—while also controlling the molecular motions at the quantum level, JILA scientists can study how the molecules scatter or interact with each other quantum mechanically. They were able to observe how the quantum effects of the molecule as a whole dictate reactivity. This new window into molecular behavior has allowed the observation of long-range interactions in which quantum mechanics determines whether two molecules should come together to react or stay apart. Thus the JILA work pushes the field in new directions and expands the standard conception of chemistry.
The JILA quantum chemistry experiments were performed with a gas containing up to 1 trillion molecules per cubic centimeter at temperatures of a few hundred billionths of a Kelvin (nanokelvins) above absolute zero (minus 273 degrees Celsius or minus 459 degrees Fahrenheit). Each molecule consists of one potassium atom and one rubidium atom. The molecules have a negative electric charge on the potassium side and a positive charge on the rubidium side, so they can be controlled with electric fields.
By measuring how many molecules are lost over time from a gas confined inside a laser-based optical trap, at different temperatures and under various other conditions, the JILA team found evidence of heat-producing chemical reactions in which the molecules must have exchanged atoms, broken chemical bonds, and forged new bonds. Theoretical calculations of long-range quantum effects agree with the experimental observations.
In conventional chemistry at room temperature, molecules may collide and react to form different compounds, releasing heat. In JILA's ultracold experiments, quantum mechanics reigns and the molecules spread out as ethereal rippling waves instead of acting as barbell-like solid particles. They do not collide in the conventional sense. Rather, as their quantum mechanical wave properties overlap, the molecules sense each other from as much as 100 times farther apart than would be expected under ordinary conditions. At this distance the molecules either scatter from one another or, if quantum conditions are right, swap atoms. Scientists expect to be able to control long-range interactions by creating molecules with specific internal states and "tuning" their reaction energies with electric and magnetic fields.
The JILA team produced a highly dense molecular gas and found that, although molecules move slowly at ultralow temperatures, reactions can occur very quickly. However, reactions can be suppressed using quantum mechanics. For instance, a cloud of molecules in the lowest-energy electronic, vibrational and rotational states reacts differently if the nuclear spins of some molecules are flipped. If a cloud of molecules is divided 50/50 into two different nuclear spin states, reactions proceed 10 to 100 times faster than if all molecules possess the same spin state. Thus, by purifying the gas (by preparing all molecules in the same spin state), scientists can deliberately suppress reactions.
The JILA experimental team attributes these results to the fact the molecules are fermions, one of two types of quantum particles found in nature. (Bosons are the second type.) Two identical fermions cannot be in the same place at the same time. This quantum behavior of fermions manifests as a suppression of the chemical reaction rate in the ultralow temperature gas. That is, molecules with identical nuclear spins are less likely to approach each other and react than are particles with opposite spins.
The JILA research is supported by NIST, the National Science Foundation and the Department of Energy.
As a non-regulatory agency of the U.S. Department of Commerce, NIST promotes U.S. innovation and industrial competitiveness by advancing measurement science, standards and technology in ways that enhance economic security and improve our quality of life.
* S. Ospelkaus, K.K. Ni, D. Wang, M.H.G. de Miranda, B. Neyenhuis, G. Quéméner, P.S. Julienne, J.L. Bohn, D.S. Jin, and J. Ye. 2010. Quantum-State Controlled Chemical Reactions of Ultracold KRb Molecules. Science. Feb. 12.
####
About NIST
From automated teller machines and atomic clocks to mammograms and semiconductors, innumerable products and services rely in some way on technology, measurement, and standards provided by the National Institute of Standards and Technology.
Founded in 1901, NIST is a non-regulatory federal agency within the U.S. Department of Commerce. NIST's mission is to promote U.S. innovation and industrial competitiveness by advancing measurement science, standards, and technology in ways that enhance economic security and improve our quality of life.
For more information, please click here
Contacts:
Laura Ost
(303) 497-4880
University of Colorado Contact:
Peter Caughey
(303) 492-4007
Copyright © NIST
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Alliances/Trade associations/Partnerships/Distributorships
![]() Chicago Quantum Exchange welcomes six new partners highlighting quantum technology solutions, from Chicago and beyond September 23rd, 2022
Chicago Quantum Exchange welcomes six new partners highlighting quantum technology solutions, from Chicago and beyond September 23rd, 2022
![]() University of Illinois Chicago joins Brookhaven Lab's Quantum Center June 10th, 2022
University of Illinois Chicago joins Brookhaven Lab's Quantum Center June 10th, 2022
Quantum nanoscience
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Optically trapped quantum droplets of light can bind together to form macroscopic complexes March 8th, 2024
Optically trapped quantum droplets of light can bind together to form macroscopic complexes March 8th, 2024
![]() Bridging light and electrons January 12th, 2024
Bridging light and electrons January 12th, 2024
![]() 'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








