Home > Press > UT Knoxville and ORNL researchers turn algae into high-temperature hydrogen source
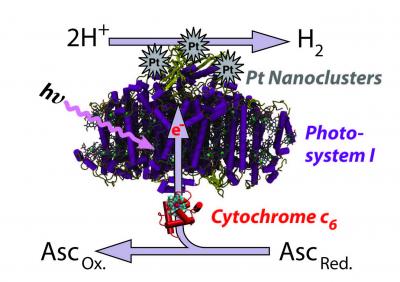 |
| This image shows the process by which Photosystem I in thermophilic blue-green algae can be catalyzed by platinum to produce a sustainable source of hydrogen. The system was highlighted in a paper by University of Tennessee, Knoxville research Barry Bruce, et al. in Nature Nanotechnology. Credit: Barry D. Bruce/University of Tennessee, Knoxville |
Abstract:
Platinum-catalyzed photosynthetic process creates high-yield sustainable source of hydrogen
UT Knoxville and ORNL researchers turn algae into high-temperature hydrogen source
Knoxville, TN | Posted on November 14th, 2009In the quest to make hydrogen as a clean alternative fuel source, researchers have been stymied about how to create usable hydrogen that is clean and sustainable without relying on an intensive, high-energy process that outweighs the benefits of not using petroleum to power vehicles.
New findings from a team of researchers from the University of Tennessee, Knoxville, and Oak Ridge National Laboratory, however, show that photosynthesis - the process by which plants regenerate using energy from the sun - may function as that clean, sustainable source of hydrogen.
The team, led by Barry Bruce, a professor of biochemistry and cellular and molecular biology at UT Knoxville, found that the inner machinery of photosynthesis can be isolated from certain algae and, when coupled with a platinum catalyst, is able to produce a steady supply of hydrogen when exposed to light.
The findings are outlined in this week's issue of the journal Nature Nanotechnology.
Bruce, who serves as the associate director for UT Knoxville's Sustainable Energy and Education Research Center, notes that we already get most of our energy from photosynthesis, albeit indirectly.
The fossil fuels of today were once, millions of years ago, energy-rich plant matter whose growth also was supported by the sun via the process of photosynthesis. There have been efforts to shorten this process, namely through the creation of biomass fuels that harvest plants and covert their hydrocarbons into ethanol or biodiesel.
"Biofuel as many people think of it now -- harvesting plants and converting their woody material into sugars which get distilled into combustible liquids -- probably cannot replace gasoline as a major source of fuel," said Bruce. "We found that our process is more direct and has the potential to create a much larger quantity of fuel using much less energy, which has a wide range of benefits."
A major benefit of Bruce's method is that it cuts out two key middlemen in the process of using plants' solar conversion abilities. The first middle man is the time required for a plant to capture solar energy, grow and reproduce, then die and eventually become fossil fuel. The second middle man is energy, in this case the substantial amount of energy required to cultivate, harvest and process plant material into biofuel. Bypassing these two options and directly using the plant or algae's built-in solar system to create clean fuel can be a major step forward.
Other scientists have studied the possibility of using photosynthesis as a hydrogen source, but have not yet found a way to make the reaction occur efficiently at the high temperatures that would exist in a large system designed to harness sunlight.
Bruce and his colleagues found that by starting with a thermophilic blue-green algae, which favors warmer temperatures, they could sustain the reaction at temperatures as high as 55 degrees C, or 131 degrees F. That is roughly the temperature in arid deserts with high solar irradiation, where the process would be most productive. They also found the process was more than 10 times more efficient as the temperature increased.
"As both a dean and a chemist, I am very impressed with this recent work by Professor Bruce and his colleagues," said Bruce Bursten, dean of UT Knoxville's College of Arts and Sciences. "Hydrogen has the potential to be the cleanest fuel alternative to petroleum, with no greenhouse gas production, and we need new innovations that allow for hydrogen to be readily produced from non-hydrocarbon sources. Professor Bruce and his team have provided a superb example of how excellence in basic research can contribute significantly to technological and societal advances."
###
Co-authors on the paper along with Bruce include Infeyinwa Iwuchukwu, a UT Knoxville graduate student in chemical and biomolecular engineering; Michael Vaughn, a research technician; Natalie Myers, a UT Knoxville graduate student in microbiology; Hugh O'Neill, a UT Knoxville-ORNL research professor and Paul Frymier, a UT Knoxville professor of chemical and biomolecular engineering.
####
About University of Tennessee at Knoxville
UT Knoxville buzzes with energy, ideas, and optimism. Great professors and students from throughout the world live and work in a friendly, safe campus community located in scenic East Tennessee. The campus and its signature "Hill" lure students with green space, nearby lakes, and vistas of the Great Smoky Mountains National Park.
Students enjoy provocative speakers, great entertainers and artists, a first-class research library, a technology-rich infrastructure, great local music and recreation, nationally competitive athletic teams, and abundant opportunities for community service.
The university is a co-manager with Battelle of the nearby Oak Ridge National Laboratory. Faculty and students experience unparalleled research and learning opportunities at the Department of Energy's largest science and energy lab.
For more information, please click here
Contacts:
Jay Mayfield
865-974-9409
Copyright © Eurekalert
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Environment
![]() Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
![]() Onion-like nanoparticles found in aircraft exhaust May 14th, 2025
Onion-like nanoparticles found in aircraft exhaust May 14th, 2025
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Fuel Cells
![]() Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Alliances/Trade associations/Partnerships/Distributorships
![]() Chicago Quantum Exchange welcomes six new partners highlighting quantum technology solutions, from Chicago and beyond September 23rd, 2022
Chicago Quantum Exchange welcomes six new partners highlighting quantum technology solutions, from Chicago and beyond September 23rd, 2022
![]() University of Illinois Chicago joins Brookhaven Lab's Quantum Center June 10th, 2022
University of Illinois Chicago joins Brookhaven Lab's Quantum Center June 10th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








