Home > Press > Discovery of an Unexpected Boost for Solar Water-Splitting Cells
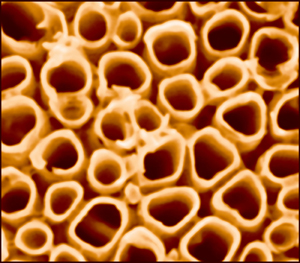 |
| Scanning electron microscope image of typical titania nanotubes for a photocatalytic cell to produce hydrogen gas from water. Nanotubes average roughly 90-100 nanometers in diameter. Follow this link for an image showing schematic of an experimental photocatlytic cell.
Credit: Menon, Northeastern University |
Abstract:
A research team from Northeastern University and the National Institute of Standards and Technology (NIST) has discovered, serendipitously, that a residue of a process used to build arrays of titania nanotubes—a residue that wasn't even noticed before this—plays an important role in improving the performance of the nanotubes in solar cells that produce hydrogen gas from water. Their recently published results* indicate that by controlling the deposition of potassium on the surface of the nanotubes, engineers can achieve significant energy savings in a promising new alternate energy system.
Discovery of an Unexpected Boost for Solar Water-Splitting Cells
Gaithersburg, MD | Posted on April 22nd, 2009Titania (or titanium dioxide) is a versatile chemical compound best known as a white pigment. It's found in everything from paint to toothpastes and sunscreen lotions. Thirty-five years ago Akira Fujishima startled the electrochemical world by demonstrating that it also functioned as a photocatalyst, producing hydrogen gas from water, electricity and sunlight. In recent years, researchers have been exploring different ways to optimize the process and create a commercially viable technology that, essentially, transforms cheap sunlight into hydrogen, a pollution-free fuel that can be stored and shipped.
Increasing the available surface area is one way to boost a catalyst's performance, so a team at Northeastern has been studying techniques to build tightly packed arrays of titania nanotubes, which have a very high surface to volume ratio. They also were interested in how best to incorporate carbon into the nanotubes, because carbon helps titania absorb light in the visible spectrum. (Pure titania absorbs in the ultraviolet region, and much of the ultraviolet is filtered by the atmosphere.)
This brought them to the NIST X-ray spectroscopy beamline at the National Synchrotron Light Source (NSLS)**. The NIST facility uses X-rays that can be precisely tuned to measure chemical bonds of specific elements, and is at least 10 times more sensitive than commonly available laboratory instruments, allowing researchers to detect elements at extremely low concentrations. While making measurements of the carbon atoms, the team noticed spectroscopic data indicating that the titania nanotubes had small amounts of potassium ions strongly bound to the surface, evidently left by the fabrication process, which used potassium salts. This was the first time the potassium has ever been observed on titania nanotubes; previous measurements were not sensitive enough to detect it.
The result was mildly interesting, but became much more so when the research team compared the performance of the potassium-bearing nanotubes to similar arrays deliberately prepared without potassium. The former required only about one-third the electrical energy to produce the same amount of hydrogen as an equivalent array of potassium-free nanotubes. "The result was so exciting," recalls Northeastern physicist Latika Menon, "that we got sidetracked from the carbon research." Because it has such a strong effect at nearly undetectable concentrations, Menon says, potassium probably has played an unrecognized role in many experimental water-splitting cells that use titania nanotubes, because potassium hydroxide is commonly used in the cells. By controlling it, she says, hydrogen solar cell designers could use it to optimize performance.
* C. Richter, C. Jaye, E. Panaitescu, D.A. Fischer, L.H. Lewis, R.J. Willey and L. Menon. Effect of potassium adsorption on the photochemical properties of titania nanotube arrays. J. Mater. Chem., published online as an Advanced Article, March 27, 2009. DOI: 10.1039/b822501j
** The NSLS is part of the Department of Energy's Brookhaven National Laboratory.
####
For more information, please click here
Contacts:
Michael Baum
(301) 975-2763
Copyright © NIST
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Nanotubes/Buckyballs/Fullerenes/Nanorods/Nanostrings
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
![]() Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Research partnerships
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() 'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








