Home > Press > Brown Chemists Create More Efficient Palladium Fuel Cell Catalysts
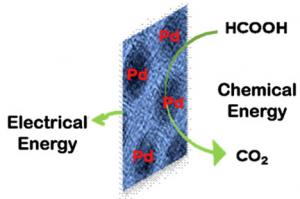 |
| A platinum alternative Brown researchers have found a way to create a larger active surface area with palladium nanoparticles to catalyze energy-producing reactions in a fuel cell. |
Abstract:
Two Brown University chemists have overcome a challenge to fuel cell reactions using palladium catalysts. The scientists produced palladium nanoparticles with about 40 percent greater active surface area than commercially available palladium particles, and the nanoparticles remain intact four times longer. Results appear in the online edition of the Journal of the American Chemical Society.
Brown Chemists Create More Efficient Palladium Fuel Cell Catalysts
Providence, RI | Posted on March 31st, 2009Even small devices need power, and much of that juice comes from fuel cells. As these devices become even smaller, the rush is on to find more efficient ways to power them.
In the last several years, scientists have discovered that palladium, a metal, is a strong candidate for providing that initial boost that helps fuel cells go. Palladium is far cheaper than another popular fuel cell catalyst, platinum, and it's more abundant.
But researchers have wrestled with creating palladium nanoparticles with enough active surface area to make catalysis efficient in fuel cells while preventing particles from clumping together during the chemical processes that convert a fuel source to electricity. Two Brown University chemists have found a way to overcome those challenges.
The scientists report in the online edition of the Journal of the American Chemical Society that they have produced palladium nanoparticles with about 40 percent greater surface area than commercially available palladium particles. The Brown catalysts also remain intact four times longer than what's currently available.
"This approach is very novel. It works," said Vismadeb Mazumder, a graduate student who joined chemistry professor Shouheng Sun on the paper. "It's two times as active, meaning you need half the energy to catalyze. And it's four times as stable."
Mazumder and Sun created palladium nanoparticles 4.5 nanometers in size. They attached the nanoparticles to a carbon platform at the anode end of a direct formic acid fuel cell. The researchers then did something new: They used weak binding amino ligands to keep the palladium nanoparticles separate and at the same size as they're attached to the carbon platform. By keeping the particles separate and uniform in size, they increased the available surface area on the platform and raised the efficiency of the fuel cell reaction.
"It just works better," Sun said.
What's also special about the ligands is that they can be "washed" from the carbon platform without jeopardizing the integrity of the separated palladium nanoparticles. This is an important step, Mazumder emphasized, because previous attempts to remove binding ingredients have caused the particles to lose their rigid sizes and clump together, which gums up the reaction.
The Brown team said in experiments lasting 12 hours, their catalysts lost 16 percent of its surface area, compared to a 64-percent loss in surface area in commercial catalysts.
"We managed to ebb the decay of our catalyst by our approach," said Mazumder, who is in his second year in Sun's lab. "We made high-quality palladium nanoparticles, put them efficiently on a support, then removed them from the stabilizers efficiently without distorting catalyst quality."
The Brown scientists now are looking at various palladium-based catalysts with enhanced activity and stability for future fuel cell applications.
"We want to make it cheaper with analogous activity," Mazumder said.
The research was funded by the Division of Materials Research of the National Science Foundation and a Brown seed fund.
####
For more information, please click here
Contacts:
Media Relations
Brown University / Box R
38 Brown Street
Providence, RI 02912
401-863-2476
Copyright © Brown University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








