Home > Press > Recycling a Greenhouse Gas for High-Energy Fuel
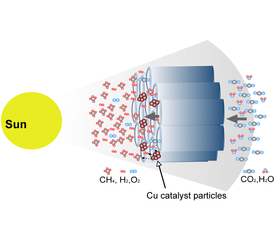 |
| Grimes Group A proposed flow-through reactor for more efficient conversion of CO2 to methane. |
Abstract:
A team of researchers at Penn State has come up with an ingenious method of turning captured CO2 into methane using the energy of the sun.
Recycling a Greenhouse Gas for High-Energy Fuel
University Park, PA | Posted on February 27th, 2009Fossil fuel use, ranging from electricity generating power plants to automobiles, pumps billions of tons of greenhouse gases into the atmosphere annually, changing the climate in ways that are likely to be detrimental to future generations. The rising use of fossil fuels, driven by population growth and rising standards of living across the globe, adds to the urgency of finding a solution to the problem of rapidly increasing atmospheric carbon dioxide, the major greenhouse gas. At Penn State, a team of researchers led by Craig Grimes has come up with an ingenious method of turning captured CO2 into methane, a combustible fuel, using the energy of the sun.
Writing in Nano Letters (Volume 9, 2009, pp 731-737), Grimes and his team describe a highly efficient photocatalyst that can yield significant amounts of methane, other hydrocarbons, and hydrogen in a simple, inexpensive process. The team used arrays of nitrogen-doped titania nanotubes sputter-coated with an ultrathin layer of a platinum and/or copper co-catalyst(s). The titania captures high energy ultraviolet wavelengths, while the copper shifts the bandgap into the visible wavelengths to better utilize the part of the solar spectrum where most of the energy lies. In addition, the thin-walled nanotubes increase the transport ability of the charge carriers by reducing the chance for recombination of the electron with the hole.
The nanotube arrays were placed inside a stainless steel chamber filled with carbon dioxide infused with water vapor. The chamber was then set outdoors in sunlight; after a few hours the team measured the amount of CO2 converted into useful fuels. The results showed 160 µL of methane per hour per gram of nanotubes, a conversion rate approximately 20 times higher than previous efforts done under laboratory conditions using pure UV light.
"Copper oxide and titanium dioxide are common materials," Grimes says. "We can tune the reaction using platinum nanoparticles or ideally other, less expensive catalysts." Grimes believes that the conversion process can readily be improved by several orders of magnitude, which could make the process economically feasible.
"You could have a small scale solar condenser and a concentrated source of CO2 in a closed loop cycle to make a portable fuel. It's a good way of storing energy for when the sun goes down," he suggests. Inexpensive solar concentrators could improve the process, as the photocatalytic CO2 conversion appears to scale with the intensity of sunlight.
Capturing CO2 at source points, such as fossil fuel (coal, natural gas, etc.)-burning power plants, and turning it into a transportation fuel in a cheap, sunlight-driven process could dramatically improve the economics of CO2 capture. "Then maybe we could figure out how to capture and reuse the CO2 in our vehicles and none of it would go back into the atmosphere," Grimes proposes.
Future research will look into increasing conversion rates by modifying the co-catalysts and changing the reactor design from a batch reactor to a flow-through photocatalytic design. "We are now reaching for low hanging fruit," Grimes says. "There is plenty of opportunity for dramatic improvements."
The article authors are Materials Research Institute scientists Oomman K. Varghese, Ph.D. and Maggie Paulose, Ph.D.; Thomas J. LaTempa, a graduate student in the Department of Electrical Engineering; and Craig A. Grimes, Ph.D., a professor of electrical engineering and materials science and engineering, as well as a faculty member in the Materials Research Institute at Penn State. Visit the Materials Research Institute online at www.mri.psu.edu.
####
For more information, please click here
Contacts:
Walt Mills
814-865-0285
PI
Craig Grimes
814-865-9142
Copyright © Newswise
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








