Home > Press > New Catalyst Paves the Path for Ethanol-Powered Fuel Cells
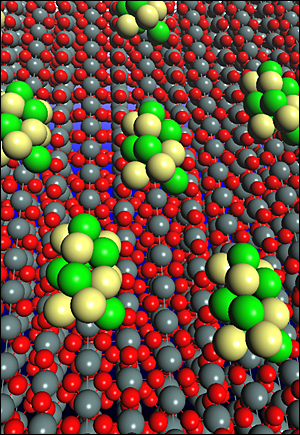 |
| Model of a ternary electrocatalyst for ethanol oxidation consisting of platinum-rhodium clusters on a surface of tin dioxide. This catalyst can split the carbon-carbon bond and oxidize ethanol to carbon dioxide within fuel cells. |
Abstract:
A team of scientists at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory, in collaboration with researchers from the University of Delaware and Yeshiva University, has developed a new catalyst that could make ethanol-powered fuel cells feasible. The highly efficient catalyst performs two crucial, and previously unreachable steps needed to oxidize ethanol and produce clean energy in fuel cell reactions. Their results are published online in the January 25, 2009 edition of Nature Materials.
New Catalyst Paves the Path for Ethanol-Powered Fuel Cells
UPTON, NY | Posted on January 25th, 2009Like batteries that never die, hydrogen fuel cells convert hydrogen and oxygen into water and, as part of the process, produce electricity. However, efficient production, storage, and transport of hydrogen for fuel cell use is not easily achieved. As an alternative, researchers are studying the incorporation of hydrogen-rich compounds, for example, the use of liquid ethanol in a system called a direct ethanol fuel cell.
"Ethanol is one of the most ideal reactants for fuel cells," said Brookhaven chemist Radoslav Adzic. "It's easy to produce, renewable, nontoxic, relatively easy to transport, and it has a high energy density. In addition, with some alterations, we could reuse the infrastructure that's currently in place to store and distribute gasoline."
A major hurdle to the commercial use of direct ethanol fuel cells is the molecule's slow, inefficient oxidation, which breaks the compound into hydrogen ions and electrons that are needed to generate electricity. Specifically, scientists have been unable to find a catalyst capable of breaking the bonds between ethanol's carbon atoms.
But at Brookhaven, scientists have found a winner. Made of platinum and rhodium atoms on carbon-supported tin dioxide nanoparticles, the research team's electrocatalyst is capable of breaking carbon bonds at room temperature and efficiently oxidizing ethanol into carbon dioxide as the main reaction product. Other catalysts, by comparison, produce acetalhyde and acetic acid as the main products, which make them unsuitable for power generation.
"The ability to split the carbon-carbon bond and generate CO2 at room temperature is a completely new feature of catalysis," Adzic said. "There are no other catalysts that can achieve this at practical potentials."
Structural and electronic properties of the electrocatalyst were determined using powerful x-ray absorption techniques at Brookhaven's National Synchrotron Light Source, combined with data from transmission electron microscopy analyses at Brookhaven's Center for Functional Nanomaterials. Based on these studies and calculations, the researchers predict that the high activity of their ternary catalyst results from the synergy between all three constituents - platinum, rhodium, and tin dioxide - knowledge that could be applied to other alternative energy applications.
"These findings can open new possibilities of research not only for electrocatlysts and fuel cells but also for many other catalytic processes," Adzic said.
Next, the researchers will test the new catalyst in a real fuel cell in order to observe its unique characteristics first hand.
This work is supported by the Office of Basic Energy Sciences within DOE's Office of Science.
####
About Brookhaven National Laboratory
One of ten national laboratories overseen and primarily funded by the Office of Science of the U.S. Department of Energy (DOE), Brookhaven National Laboratory conducts research in the physical, biomedical, and environmental sciences, as well as in energy technologies and national security. Brookhaven Lab also builds and operates major scientific facilities available to university, industry and government researchers. Brookhaven is operated and managed for DOE’s Office of Science by Brookhaven Science Associates, a limited-liability company founded by Stony Brook University, the largest academic user of Laboratory facilities, and Battelle, a nonprofit, applied science and technology organization.
For more information, please click here
Contacts:
Kendra Snyder
(631) 344-8191
or
Mona S. Rowe
(631) 344-5056
Copyright © Brookhaven National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








