Home > Press > Structural ‘snapshots’ of a protein implicated in Alzheimer’s disease
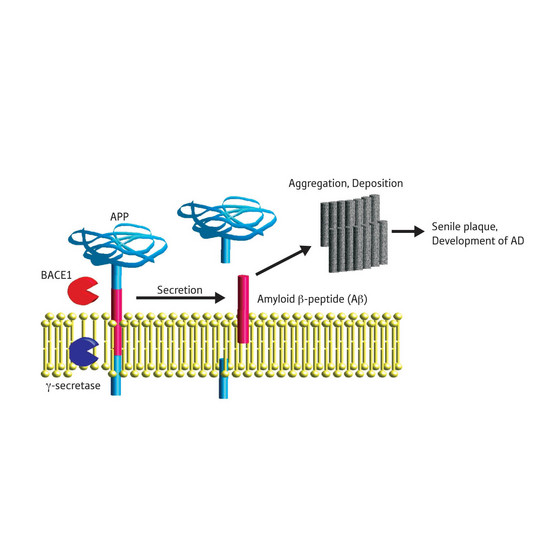 |
| Figure 1: Schematic depicting generation of Aβ from APP. γ-secretase is another enzyme involved in the process. AD, Alzheimer’s disease. |
Abstract:
New experiments reveal detailed physical features of a protein thought to exacerbate the pathology of Alzheimer's disease
Structural ‘snapshots’ of a protein implicated in Alzheimer’s disease
Japan | Posted on September 25th, 2008A recent study describes the structure of the active form of BACE1, which is an enzyme implicated in Alzheimer's disease. BACE1 cleaves amyloid precursor protein (APP), thereby releasing amyloid β peptide (Aβ), the primary component of amyloid plaques found in the brains of patients with Alzheimer's disease (Fig. 1).
As amyloid plaques are thought by many to inflict brain cell damage that results in Alzheimer's disease, efforts are under way to design drugs to inhibit the activity of BACE1. Complicating these efforts is the fact that BACE1 seems to cleave APP in vesicles called endosomes, which sport a pH much more acidic than that of other areas of the cell or the extracellular fluid.
Structures of several BACE1 complexes have been solved using a technique called x-ray crystallography, wherein structural information is gleaned from x-rays diffracted from crystallized versions of proteins. However, never before has a structural view of active BACE1 been available. In a paper recently published in Molecular and Cellular Biology, Nobuyuki Nukina and colleagues from the RIKEN Brain Science Institute in Wako and the RIKEN SPring-8 Center in Harima present and analyze crystals of active BACE11.
To identify conditions in which crystallized BACE1 is active, the researchers soaked BACE1 crystals in acidic (pH 4.0, 4.5 and 5.0) and neutral (pH 7.0) solutions, together with synthetic APP peptides engineered to fluoresce after cleavage. In agreement with data localizing BACE1 activity to acidic endosomes, crystallized BACE1 cleaved APP at acidic but not neutral pH.
photo
Video 1: Shape changes associated with activation of BACE1.
High resolution video and legend
Comparative analyses revealed substantial differences in the shape of BACE1 crystals soaked in acidic and neutral solutions, suggesting that BACE1 undergoes structural rearrangements during activation (Vid. 1). Most notable was the position of the ‘flap' covering the active site of BACE1, which was open and closed in acidic and neutral crystals, respectively. Also observed were marked changes in the shape of the BACE1 site at which the substrate—in this case, APP—binds.
Binding of a water molecule—thought to be important in the chemical reaction through which BACE1 cleaves APP—became weaker as the pH was lowered. Whether BACE1 exists as a mix of hydrated active and dehydrated inactive forms in endosomes remains unclear.
These findings highlight the importance of considering environmental factors such as pH in structure-based design of enzyme inhibitors. "The structure of the active form of BACE1 identified here should be used for developing drugs to regulate Aβ production," says Nukina.
Reference
1. Shimizu, H., Tosaki, A., Kaneko, K., Hisano, T., Sakurai, T. & Nukina, N. Crystal structure of an active form of BACE1, an enzyme responsible for amyloid β protein production. Molecular and Cellular Biology 28, 3663-3671 (2008).
####
For more information, please click here
Copyright © Riken
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Nanomedicine
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
![]() Researchers develop artificial building blocks of life March 8th, 2024
Researchers develop artificial building blocks of life March 8th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








