Home > Press > Hydrogen atom trapped in unique cage
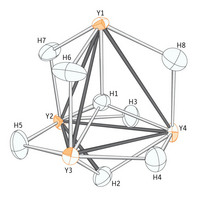 |
| Figure 1: The hydrogen atom (H1) nestles perfectly between four yttrium atoms (orange) in the unusual organometallic compound.
Reproduced with permission from Ref. 2 © American Chemical Society |
Abstract:
Unusual compound sees hydrogen bonded to four metal atoms
Hydrogen atom trapped in unique cage
Japan | Posted on July 3rd, 2008RIKEN scientists, along with an international team of co-workers based in the US, UK and France, have spotted a hydrogen atom simultaneously bonding to four metal atoms, a highly unusual arrangement in this particular class of chemical compounds. The research may help to understand the structure of materials that could store hydrogen more efficiently.
Hydrogen normally prefers to bond with just one other atom. However, it can be persuaded into polygamy by surrounding it with metal atoms that form a cage-like cluster.
Certain compounds of uranium or thorium are already known to host four-coordinate hydrogen atoms. But these compounds' structures and properties are usually difficult to control or modify.
Conversely, the behavior of organometallic compounds—which possess organic (carbon-based) molecules as supporting groups around the metal atoms—can be easily controlled by changing the organic groups, allowing the compound's properties to be fine-tuned.
Zhaomin Hou and colleagues from RIKEN's Advanced Science Institute in Wako had previously prepared a series of organometallic compounds containing four metal atoms with eight hydrogen atoms and studied their structures by X-ray diffraction experiments1.
Now, in collaboration with the international team of co-workers, Hou and colleagues have studied a compound based on the metal yttrium using neutron diffraction2, a more precise technique which fires a stream of the neutral particles through a crystal of the compound and uses their subsequent trajectory to calculate the precise arrangement of atoms (Fig. 1). Two crystals, both smaller than the head of a match, were analyzed at facilities in the UK and France.
The team found that hydrogen atoms in different parts of the same compound display a range of three different bonding modes—hooking up with either two, three or four other atoms. The distance between the four-coordinate hydrogen atom and its yttrium neighbors is also surprisingly small: strong evidence that hydrogen fits tightly into the cage made by the four metal atoms.
Hou says that "studies like this will increase our understanding and appreciation of the element hydrogen and its chemistry." But it may also have practical applications. "The increasing interest in hydrogen as a fuel warrants careful studies into the structural chemistry of this most ubiquitous element," he says, adding that there are very few high-precision studies on compounds containing multiply bonded hydrogen.
The team is now studying similar compounds containing two or more different types of metals, which are expected to have unique structures and properties of their own.
Reference
1. Hou, Z., Nishiura, M. & Shima, T. Synthesis and reactions of polynuclear polyhydrido rare earth metal complexes containing "(C5Me4SiMe3)LnH2" units: A new frontier in rare earth metal hydride chemistry. European Journal of Inorganic Chemistry 18, 2535-2545 (2007).
2. Yousufuddin, M., Gutmann, M.J., Baldamus, J., Tardif, O., Hou, Z., Mason, S.A., McIntyre, G.J. & Bau, R. Neutron diffraction studies on a 4-coordinate hydrogen atom in an yttrium cluster. Journal of the American Chemical Society 130, 3888-3891 (2008).
####
For more information, please click here
Copyright © Riken
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Research partnerships
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() 'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








