Home > Press > Search for future hydrogen storage materials extends to investigation of hydrogen interactions with metal nanoparticles
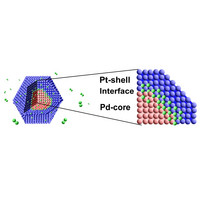 |
| Figure 1: A schematic depiction of hydrogen storage of palladium (Pd) and platiunum (Pt) nanoparticles (green, hydrogen; red, Pd; blue Pt).
Reproduced with permission from Ref. 1 © 2008 by the American Chemical Society |
Abstract:
The environmental impact of the use of hydrocarbons as fuels has led to a global search for cleaner energy sources. Hydrogen offers a greener alternative for transportation fuels, but a critical issue is the requirement of a safe and reliable hydrogen storage medium. Nanoparticles have advantages over bulk materials for hydrogen storage applications: they have a larger solid/gas interface area and shorter hydrogen diffusion paths, yielding potentially faster kinetics for gas absorption and desorption.
Search for future hydrogen storage materials extends to investigation of hydrogen interactions with metal nanoparticles
Japan | Posted on April 26th, 2008In two recent communications published in the Journal of the American Chemical Society, Masaki Takata from the SPring-8 Centre, Harima, and his colleagues, including Hiroshi Kitagawa from Kyushu University, explore the hydrogen absorption and desorption behavior of palladium nanoparticles and of palladium core-platinum shell nanoparticles.
In the first communication1, the researchers address whether core-shell nanoparticles made of two metals store hydrogen. The team prepared structures with crystalline palladium cores of 6 nm diameter and crystalline platinum shells of thickness around 2 nm, and then characterized them using a variety of techniques.
Pressure-composition isotherms showed that the core-shell nanoparticles absorbed the same amount of hydrogen as homogenous palladium nanoparticles. Takata, Kitagawa and colleagues then performed solid state nuclear magnetic resonance (NMR) measurements with deuterium, a hydrogen isotope, to identify the absorption site of hydrogen. Surprisingly, they have found that while deuterium was dispersed in both palladium and platinum lattices, it was concentrated in the boundary region between the core and the shell (Fig. 1).
Palladium nanoparticles do not demonstrate complete reversibility in their hydrogen uptake and release, in contrast to their bulk counterparts. Takata, Kitagawa and colleagues explored this hysteresis in their second communication2. Using x-ray diffraction, they have found that the lattice constant of palladium nanoparticles of 6 nm diameter increases with exposure to increased hydrogen pressures. However, on evacuation of the hydrogen, the lattice does not return to its original value; it remains slightly larger.
Then, again using solid state NMR measurements with deuterium, the researchers have found that some deuterium atoms remained within the palladium lattice after evacuation of ‘free' deuterium from the system. They suggest that hydrogen atoms are trapped firmly within the lattice, which expands the crystal lattice, and hence lattice constant, of palladium. This, they say, explains why hydrogen absorption in these materials is not completely reversible.
The researchers conclude that their work provides a new understanding of the interactions between hydrogen and ‘nano-structured' solids, and could contribute to the development of practical hydrogen-storage materials.
Reference
1. Kobayashi, H., Yamauchi, M., Kitagawa, H., Kubota, Y., Kato, K. & Takata, M. Hydrogen absorption in the core/shell interface of Pd/Pt nanoparticles. Journal of the American Chemical Society 130, 1818-1819 (2008).
2. Kobayashi, H., Yamauchi, M., Kitagawa, H., Kubota, Y., Kato, K. & Takata, M. On the nature of strong hydrogen atom trapping inside Pd nanoparticles. Journal of the American Chemical Society 130, 1828-1829 (2008). | article |
####
About Riken Research
RIKEN is one of Japan’s largest research organisations with institutes and centres in various locations in Japan (see http://www.riken.jp/engn/r-world/link/index.html). RIKEN’s 3000+ researchers publish several hundred research articles in top scientific and technical journals every year across a broad spectrum of disciplines in physics, chemistry, biology, medicine, earth science and in many areas of technology, and the number of articles is growing year on year.
For more information, please click here
Copyright © Riken Research
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








