Home > Press > New nanoparticle catalyst brings fuel-cell cars closer to showroom
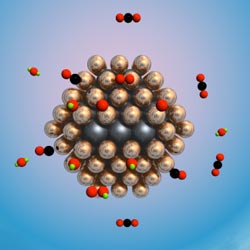 |
| UW-Madison and University of Maryland researchers developed a new type of catalyst by surrounding a nanoparticle of ruthenium with one to two layers of platinum atoms. The result is a robust room-temperature catalyst that dramatically improves a key hydrogen purification reaction and leaves more hydrogen available to make energy in the fuel cell. |
Abstract:
A University of Wisconsin-Madison and University of Maryland (UM) team has developed a new nanotechnology-driven chemical catalyst that paves the way for more efficient hydrogen fuel-cell vehicles.
New nanoparticle catalyst brings fuel-cell cars closer to showroom
Madison, WI | Posted on March 19th, 2008Writing in this week's Advance Online Publication of Nature Materials, UW-Madison chemical and biological engineering Professor Manos Mavrikakis and UM chemistry and biochemistry Professor Bryan Eichhorn describe a new type of catalyst created by surrounding a nanoparticle of ruthenium (Ru) with one to two layers of platinum (Pt) atoms. The result is a robust room-temperature catalyst that dramatically improves a key hydrogen purification reaction and leaves more hydrogen available to make energy in the fuel cell.
One day, it could be common for fuel cells to create electricity by consuming hydrogen generated from renewable resources. For now, most of the world's hydrogen supply is derived from fossil fuels in a process called reforming.
An important step in this multistage process, called preferential oxidation of CO in the presence of hydrogen (PROX), uses a catalyst to purge hydrogen of carbon monoxide (CO) before it enters the fuel cell. CO presents a major obstacle to the practical application of fuel cells because it poisons the expensive platinum catalyst that runs the fuel cell reaction.
Attractive for transportation applications and as a battery replacement, proton exchange membrane fuel cells generate electricity using porous carbon electrodes containing a platinum catalyst separated by a solid polymer. Hydrogen fuel enters one side of the cell and oxygen enters on the opposite side. Platinum facilitates the production of protons from molecular hydrogen, and these protons cross the membrane to react with oxygen on the other side. The result is electricity with water and heat as byproducts.
A conventionally constructed catalyst combining ruthenium and platinum must be heated to 70 degrees Celsius or 158 degrees Fahrenheit in order to drive the PROX reaction, but the same elements combined as core-shell nanoparticles operate at room temperature. The lower the temperature at which catalyst activates the reactants and makes the products, the more energy is saved.
"We understand why it works," Mavrikakis says. "We know now the reason behind this marvelous behavior. The first reason is the core-cell nanostructure. This polymer-based method developed by my colleagues in Maryland allows the exact amount of an element, in this case platinum, to be placed exactly where you want it to be on specific seeds of ruthenium."
This very specific nano-architecture and composition can sustain significantly less CO on its surface than pure Pt would. Because the binding is weaker, Mavrikakis says fewer sites on the core-cell nanostructure are available to bind with CO than would occur with Pt alone. That leaves empty sites for oxygen to come in and react.
"The second reason is that there is a completely new reaction mechanism that makes this work so well," he says. "We call it hydrogen-assisted CO oxidation. It uses atomic hydrogen to attack molecular oxygen and make a hydroperoxy intermediate, which in turn, easily produces atomic oxygen. Then, atomic oxygen selectively attacks CO to produce CO2, leaving much more molecular hydrogen free to be fed to the fuel cell than pure Pt does."
While the breakthrough is important to the development of fuel-cell technology, the researchers say it's even more significant to catalysis in general.
First, the team, including graduate students Anand Nilekar of UW-Madison and Selim Alayoglu of Maryland, used theory rather than an experimental approach to zero in on ruthenium/platinum as the ideal core shell system.
Second, the nanoscale fabrication of ruthenium and platinum resulted in a different nano-architecture than when ruthenium and platinum are combined in bulk. For the field of catalysis, the pairing of these approaches could bridge the gap between surface science and catalysis opening new paths to novel and more energy-efficient materials discovery for a variety of industrially important chemical processes.
####
For more information, please click here
Contacts:
Terry Devitt
science
(608) 262-8282
Copyright © University of Wisconsin-Madison
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Automotive/Transportation
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








