Home > Press > Water flows like molasses on the nanoscale
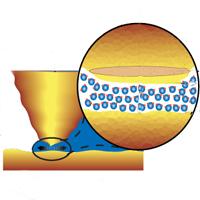 |
| Georgia Tech physicists have discovered that water behaves differently when its compressed in nano-sized channel. In these small spaces water behaves much like a solid, exhibiting high viscosity and organizing... |
Abstract:
A Georgia Tech research team has discovered that water exhibits very different properties when it is confined to channels less than two nanometers wide - behaving much like a viscous fluid with a viscosity approaching that of molasses. Determining the properties of water on the nanoscale may prove important for biological and pharmaceutical research as well as nanotechnology. The research appears in the March 15 issue of the journal Physical Review B.
Water flows like molasses on the nanoscale
Atlanta, GA | Posted on April 24th, 2007In its bulk liquid form, water is a disordered medium that flows very readily. When most substances are compressed into a solid, their density increases. But water is different; when it becomes ice, it becomes less dense. For this reason, many scientists reasoned that when water is compressed (as it is in a nanometer-sized channel), it should maintain its liquid properties and shouldn't exhibit properties that are akin to a solid. Several earlier studies came to that very conclusion - that water confined in a nano-space behaves just like water does in the macro world. Consequently, a number of scientists considered the case to be closed.
But when Georgia Tech experimental physicist Elisa Riedo and her team directly measured the force of pure water in a nanometer-sized channel, they found evidence suggesting that water was organized into layers. Riedo conducted these measurements by recording the force placed on a silicon tip of an atomic force microscope as it compressed water. The water was confined in a nanoscale thin film on top of a solid surface.
"Since water usually has a low viscosity, the force you would expect to feel as you compress it should be very small," said Riedo, assistant professor in Georgia Tech's School of Physics. "But when we did the experiment, we found that when the distance between the tip and the surface is about one nanometer, we feel a repulsive force by the water that is much stronger than what we would expect."
As the tip compresses the water even more, the repulsive force oscillates, indicating that the water molecules are forming layers. As the tip continues to increase its pressure on a layer, the layer collapses and the water flows out horizontally.
"In effect, the confined water film behaves effectively like a solid in the vertical direction by forming layers parallel to the confining tip and surface, while maintaining it's liquidity in the horizontal direction where it can flow out - resembling some phases of liquid crystals," said Uzi Landman, director of the Center for Computational Materials Science, Regents' and Institute professor, and Callaway Chair of Physics at Georgia Tech.
A theoretical physicist, Landman conducted the first-ever computer simulations of these forces for tip-confined water films and found good correspondence between his team's theoretical predictions and the experiments.
So why did Riedo and Landman's results differ from their peers? According to Landman, most previous studies on confined water were limited by technology at the time and could not directly measure the behavior in the last two nanometers. Instead they had to measure other properties and infer the forces acting in films of one nanometer thickness or less.
"If you want force, it is preferable to measure it," he said. "This is the first experiment to directly measure the force and it's the first simulation done of these forces. The fact that we have direct measurements married with theoretical results is rather conclusive."
Riedo and Landman conducted their experiments in several different environments. They found that the layering effect was more pronounced when water was placed on top of hydrophilic surfaces that allow water to wet the solid surface, such as glass. When the water was confined by hydrophobic surfaces where water tends to bead up, like graphite, the effect was still present, but less pronounced.
At the same time, Riedo's team was measuring the vertical force exerted on the tip by the confined water film, they also measured the film viscosity by measuring the lateral force. They found that when water was placed on a hydrophilic surface, the viscosity began to increase dramatically as the thickness of the confined film reached the 1.5 nanometer range. As they continued to compress the water and measure the lateral forces, the viscosity increased by a factor of 1,000 to 10,000.
On hydrophobic surfaces, they did not see such an increase in viscosity. The results of the molecular dynamics simulations support these findings, showing a dramatically decreased mobility for sub-nanometer thick water films under hydrophilic confinement.
"Water is a wonderful lubricant," said Riedo, "but it flows too easily for many applications. At the one nanometer scale, water is a viscous fluid and could be a much better lubricant."
Understanding the properties of water at this scale could also be important for biological and pharmaceutical research, especially in understanding processes that depend on hydrated ionic transport through nanoscale channels and pores.
Riedo and Landman's next steps are to introduce impurities in the water to study how that affects its properties.
Tai-De Li, Robert Szoszkiewicz and Jianping Gao also contributed to this research, which was supported by the National Science Foundation, the American Chemical Society Petroleum Foundation, the Air Force Office of Scientific Research and the U.S. Department of Energy.
####
About Georgia Institute of Technology
The Georgia Institute of Technology is one of the nation's top research universities, distinguished by its commitment to improving the human condition through advanced science and technology.
Georgia Tech's campus occupies 400 acres in the heart of the city of Atlanta, where more than 16,000 undergraduate and graduate students receive a focused, technologically based education.
Accredited by the Southern Association of Colleges and Schools (SACS), the Institute offers many nationally recognized, top-ranked programs. Undergraduate and graduate degrees are offered in the Colleges of Architecture, Engineering, Sciences, Computing, Management, and the Ivan Allen College of Liberal Arts. Georgia Tech consistently ranks among U.S. News & World Report's top ten public universities in the United States. In a world that increasingly turns to technology for solutions, Georgia Tech is using innovative teaching and advanced research to define the technological university of the 21st century.
For more information, please click here
Contacts:
David Terraso
404-385-2966
Copyright © Georgia Institute of Technology
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Water
![]() Taking salt out of the water equation October 7th, 2022
Taking salt out of the water equation October 7th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








