Home > Press > Research Shows How Water May Enhance Nanocatalysis
Abstract:
Findings could lead to fewer constraints on reaction conditions potentially leading to the development of lower cost techniques for certain industrially important catalytic reactions
Research Shows How Water May Enhance Nanocatalysis
Atlanta, GA |September 12, 2005
Researchers at the Georgia Institute of Technology have uncovered important evidence that explains how water, usually an inhibitor of catalytic reactions, can sometimes promote them. The findings could lead to fewer constraints on reaction conditions potentially leading to the development of lower cost techniques for certain industrially important catalytic reactions. The results appear in the September 6, 2005 issue of Physical Review Letters.
“Normally, in most catalytic reactions, water can stop the reaction. It kills the catalyst,” said Uzi Landman, director of the Center for Computational Materials Science, Regents’ and Institute professor and Callaway chair of physics at Georgia Tech.
And that’s a big problem because ensuring that a reaction is water-free can add to production costs. Many catalytic reactions occur at high temperatures, which evaporates the water, said Landman. “However, any time that the reaction temperature is lowered and there’s humidity unfavorable effects may occur. You hope that when you heat the reaction up that the adsorbed water will come off, but sometimes it doesn’t. Sometimes the adsorption of water leads to an irreversible modification, such as oxidation, and deactivation of the catalyst. It’s poison; it poisons the catalyst,” he said.
In the late 1980's, Japanese scientist Masatake Haruta discovered that small particles of gold (which is chemically inert in bulk form and normally not a catalyst) are chemically very reactive. He also found that water can promote this catalytic activity.
Since the late 1990's, Landman’s group has been using advanced quantum mechanical computational methods to investigate how and why nanoclusters of gold act as chemical catalysts under dry conditions. This led to certain predictions that were verified experimentally by Ulrich Heiz’s group, who is now at the Technical University of Munich.
Earlier this year, the two groups co-authored a paper in the journal Science. It showed theoretical and experimental evidence of the role of charging on the catalytic activity of gold nanoclusters made of eight atoms when they are bonded to naturally occurring oxygen vacancy defects on a magnesia surface that supports the gold. In the recent Physical Review Letters paper, the Georgia Tech group has made theoretical predictions on how a single water molecule can catalytically enhance a low-temperature reaction that turns carbon monoxide into carbon dioxide.
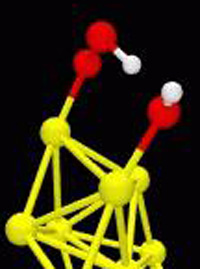
Using computer simulations, Landman and post doctoral fellow Angelo Bongiorno, found that the water molecule enhances the binding of an oxygen molecule to an eight atom gold nanocluster, either free or supported on an undefective magnesia substrate. The water molecule catalytically activates the aforementioned oxidation reaction of carbon monoxide. In the earlier studies on gold nanoclusters, defects in the support surface were required to give the gold a slight negative charge. In this latest study, the presence of a water molecule makes that requirement unnecessary.
Here’s how it works: the structure of the water molecule, H-O-H, is such that the end with the oxygen atom has a slight negative charge, while the two hydrogen atoms are positively charged. In the quantum molecular dynamics simulation, the negatively charged oxygen side of the water molecule bonds to one of the gold atoms, leaving the positively charged hydrogens of the water molecule dangling. Subsequently, an oxygen molecule (made of two oxygen atoms) binds favorably to a neighboring gold atom of the cluster and gets a slight negative charge in the process.
This results in an adsorbed slightly negatively charged oxygen molecule near one of the positively charged hydrogen atoms of the adsorbed water molecule. Since, in chemistry, (as in love) opposites attract, the two get together. So the oxygen pulls a proton (a positively charged hydrogen) from the water molecule resulting in formation of a hydroperoxyl (OOH) group and a hydroxyl (OH).
Now, this relationship can’t last because the addition of the hydrogen to the oxygen molecule to form OOH weakens the bond between the two oxygen atoms. All it takes to break that bond is a carbon monoxide molecule approaching from the gas phase, which bonds to one of the oxygens of the OOH to form carbon dioxide. This leaves the proton to return to the hydroxyl to reform the water molecule. The product carbon dioxide desorbes readily from the surface, and the left over oxygen atom stays bonded to the gold. But this single oxygen atom is very active (as singles often are) and is easily led away when another carbon monoxide comes along to bond with it to make a second carbon dioxide molecule.
“This reaction opens the door to a completely new idea; that polar molecules, like water, or molecules that are good proton donors may show us new channels of reactivity,” said Landman. “We may be able to take other catalytic reactions and use water as a promoter under some selective conditions,” added Bongiorno.
“In the future, we want to test the effect of multiple water molecules to see if there is a limit to how many water molecules can enhance reactions. In this case, we used magnesium oxide as a substrate. We’d like to know if the effect limited to that substrate or will it work with others?,” the two researchers said.
About the Georgia Institute of Technology:
The Georgia Institute of Technology is one of the nation's premiere research universities. Ranked among U.S. News & World Report's top 10 public universities, Georgia Tech educates more than 16,000 students every year through its Colleges of Architecture, Computing, Engineering, Liberal Arts, Management and Sciences. Tech maintains a diverse campus and is among the nation's top producers of women and African-American engineers. The Institute offers research opportunities to both undergraduate and graduate students and is home to more than 100 interdisciplinary units plus the Georgia Tech Research Institute. During the 2003-2004 academic year, Georgia Tech reached $341.9 million in new research award funding.
For more information, please visit www.gatech.edu
Contact:David Terraso
Media Relations Specialist
Institute Communications and Public Affairs
david.terraso@icpa.gatech.edu
404-385-2966
Copyright © Georgia Institute of Technology
If you have a comment, please Contact us.
Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Industrial
![]() Boron nitride nanotube fibers get real: Rice lab creates first heat-tolerant, stable fibers from wet-spinning process June 24th, 2022
Boron nitride nanotube fibers get real: Rice lab creates first heat-tolerant, stable fibers from wet-spinning process June 24th, 2022
![]() Nanotubes: a promising solution for advanced rubber cables with 60% less conductive filler June 1st, 2022
Nanotubes: a promising solution for advanced rubber cables with 60% less conductive filler June 1st, 2022
![]() Protective equipment with graphene nanotubes meets the strictest ESD safety standards March 25th, 2022
Protective equipment with graphene nanotubes meets the strictest ESD safety standards March 25th, 2022
![]() OCSiAl receives the green light for Luxembourg graphene nanotube facility project to power the next generation of electric vehicles in Europe March 4th, 2022
OCSiAl receives the green light for Luxembourg graphene nanotube facility project to power the next generation of electric vehicles in Europe March 4th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||