Home > Press > 'Cornell dots,' for biological tagging, imaging and optical computing
Abstract:
Move over, quantum dots. Make way for the new kids on the block -- brightly glowing nanoparticles dubbed "Cornell dots"
After quantum dots, now come glowing 'Cornell dots,' for biological tagging, imaging and optical computing
Ithaca, NY | May 19, 2005
By surrounding fluorescent dyes with a protective silica shell, Cornell University researchers have created fluorescent nanoparticles with possible applications in displays, biological imaging, optical computing, sensors and microarrays such as DNA chips. These are all applications for which quantum dots have been used or are being considered. But the new Cornell nanoparticles offer an appealing alternative because of their greater chemical inertness and reduced cost.
"People have done superb experiments with quantum dots that were not previously possible," says Ulrich Wiesner, Cornell associate professor of materials science and engineering. "Hopefully Cornell dots will serve the same purpose and offer new possibilities." There are also some interesting physics questions about how the new dots work, he adds.
Since optical microscopes can't resolve individual molecules, and electron microscopes can't be used on living organisms, biologists often tag organic molecules with fluorescent dyes in order to track their movements through biological processes, such as the action of enzymes inside a living cell. While it can't see the molecules, an optical microscope can track the bright light given off by the dye.
Quantum dots -- which have been used for the same purpose -- are tiny particles of semiconductors such as cadmium selenide that behave as if they were individual atoms: They can absorb light energy, kicking their internal electrons up to higher energy levels, then release the energy by emitting light. A quantum dot fluoresces much more brightly than a dye molecule, making it a desirable marker.
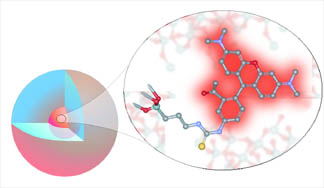
Cornell dots, also known as CU dots, are nanoparticles consisting of a core about 2.2 nanometers (nm) in diameter containing several dye molecules, surrounded by a protective silica shell, making the entire particle about 25 nm in diameter. The researchers call this a "core-shell architecture." (A nanometer is one-billionth of a meter, about three times the diameter of a silicon atom.)
Like quantum dots, CU dots are many times brighter (20-30 times) than single dye molecules in solution and resist "photobleaching," a process by which dyes in solution rapidly lose their fluorescence. CU dots can be made with a wide variety of dyes, producing a large assortment of colors.
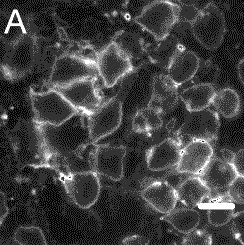
CU dots bound to immunoglobin-G antibodies attach to the surface of leukemia cells, demonstrating a possible use in biological tagging. Copyright © Cornell University |
The manufacture of CU dots and early experiments with them are described in a paper, "Bright and Stable Core-Shell Fluorescent Silica Nanoparticles," in the journal Nano Letters (Vol. 5 No. 1) by Wiesner and his Cornell colleagues Hooisweng Ow, Daniel R. Larson, Mamta Srivastava, Barbara A. Baird and Watt W. Webb .
Unlike quantum dots, CU dots are mostly chemically inert. The silica shell is silicon dioxide -- essentially glass. For use as biological markers, quantum dots are encased in a polymer shell -- a process that adds to their already high manufacturing cost. Quantum dots also contain heavy metals like cadmium that can leach through the polymer shell and disrupt the chemistry being observed.
However, Wiesner says, "Silica is benign, cheap and easy to attach, and it is totally compatible with silicon manufacturing technology. That opens enormous possibilities in the life sciences and in information technology."
The Cornell researchers tested the use of CU dots as biological markers by attaching an antibody, immunoglobin E (IgE), and observing how this combination attached to cell receptors on leukemia mast cells.
The dots also offer an intriguing physics question: Why do they fluoresce so brightly? In effect, the whole is brighter than the sum of its parts. "We have this enormous brightness, and we don't know exactly where it's coming from," Wiesner says. Several explanations have been offered. One is that the silicon shell protects the dye molecules from the solvent. A second is that dye molecules floating free can lose energy by actions other than emitting photons, but in the packed core of the particle those other actions are diminished.
The dots were created by Ow, then Wiesner's graduate student. Webb, the S.B. Eckert Professor in Engineering, and Larson, a graduate student in applied and engineering physics now at Albert Einstein College of Medicine, studied their photophysical properties. Baird, director of the Cornell Nanobiotechnology Center, and Srivastava, a postdoctoral researcher, studied the dots as labels on living cells.
The research was supported by the National Science Foundation, the state of New York and Phillip Morris USA. Quantum Dot Corp. supplied quantum dots used for comparison.
Related World Wide Web sites: Ulrich Wiesner's nanoparticle page.
Contact:
Bill Steele
(607) 255-7164
ws21@cornell.edu
Media Contact:
Office of Press Relations
(607) 255-6074
pressoffice@cornell.edu
Copyright © Cornell University
If you have a comment, please Contact us.
Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Possible Futures
![]() Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Materials/Metamaterials/Magnetoresistance
![]() First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
![]() Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
![]() A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
![]() Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Announcements
![]() Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||