Home > Press > Scientists Design Molecular System for Artificial Photosynthesis: System is designed to mimic key functions of the photosynthetic center in green plants to convert solar energy into chemical energy stored by hydrogen fuel
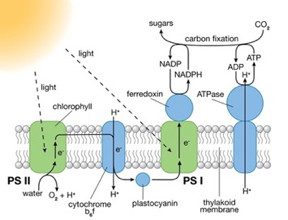 |
| Photosystems (PS) I and II are large protein complexes that contain light-absorbing pigment molecules needed for photosynthesis. PS II captures energy from sunlight to extract electrons from water molecules, splitting water into oxygen and hydrogen ions (H+) and producing chemical energy in the form of ATP. PS I uses those electrons and H+ to reduce NADP+ (an electron-carrier molecule) to NADPH. The chemical energy contained in ATP and NADPH is then used in the light-independent reaction of photosynthesis to convert carbon dioxide to sugars. |
Abstract:
Photosynthesis in green plants converts solar energy to stored chemical energy by transforming atmospheric carbon dioxide and water into sugar molecules that fuel plant growth. Scientists have been trying to artificially replicate this energy conversion process, with the objective of producing environmentally friendly and sustainable fuels, such as hydrogen and methanol. But mimicking key functions of the photosynthetic center, where specialized biomolecules carry out photosynthesis, has proven challenging. Artificial photosynthesis requires designing a molecular system that can absorb light, transport and separate electrical charge, and catalyze fuel-producing reactions-all complicated processes that must operate synchronously to achieve high energy-conversion efficiency.
Scientists Design Molecular System for Artificial Photosynthesis: System is designed to mimic key functions of the photosynthetic center in green plants to convert solar energy into chemical energy stored by hydrogen fuel
Upton, NY | Posted on June 2nd, 2017Now, chemists from the U.S. Department of Energy's (DOE) Brookhaven National Laboratory and Virginia Tech have designed two photocatalysts (materials that accelerate chemical reactions upon absorbing light) that incorporate individual components specialized for light absorption, charge separation, or catalysis into a single "supramolecule." In both molecular systems, multiple light-harvesting centers made of ruthenium (Ru) metal ions are connected to a single catalytic center made of rhodium (Rh) metal ions through a bridging molecule that promotes electron transfer from the Ru centers to the Rh catalyst, where hydrogen is produced.
[sidebar: Finding inspiration from nature
The leaves of green plants contain hundreds of pigment molecules (chlorophyll and others) that absorb light at particular wavelengths. When light of the proper wavelength strikes one of these molecules, the molecule enters an excited state. Energy from this excited state is shuttled along a chain of pigment molecules until it reaches a specific type of chlorophyll in the photosynthetic reaction center. Here, the energy is used to drive the charge-separation process required for photosynthesis to proceed. The electron "hole" left behind in the chlorophyll molecule is used for water-to-oxygen conversion. Hydrogen ions formed during the water-splitting process are eventually used for the reduction of carbon dioxide to glucose in the second stage of photosynthesis, known as the light-independent reaction.]
They compared the hydrogen-production performance and analyzed the physical properties of the supramolecules, as described in a paper published in the June 1 online edition of Journal of the American Chemical Society, to understand why the photocatalyst with six as opposed to three Ru light absorbers produces more hydrogen and remains stable for a longer period of time.
"Developing efficient molecular systems for hydrogen production is difficult because processes are occurring at different rates," said lead author Gerald Manbeck, a chemist in the artificial photosynthesis group [ https://www.bnl.gov/chemistry/AP/ ] at Brookhaven Lab. "Completing the catalytic turnover of hydrogen before the separated charges-the negatively charged light-excited electron and positive "hole" left behind after the excited molecule absorbs light energy-have a chance to recombine and wastefully produce heat is one of the major challenges."
Another complication is that two electrons are needed to produce each hydrogen molecule. For catalysis to happen, the system must be able to hold the first electron long enough for the second to show up. "By building supramolecules with multiple light absorbers that may work independently, we are increasing the probability of using each electron productively and improving the molecules' ability to function under low light conditions," said Manbeck.
Manbeck began making the supramolecules at Virginia Tech in 2012 with the late Karen Brewer, coauthor and his postdoctoral advisor. He discovered that the four-metal (tetrametallic) system with three Ru light-absorbing centers and one Rh catalytic center yielded only 40 molecules of hydrogen for every catalyst molecule and ceased functioning after about four hours. In comparison, the seven-metal (heptametallic) system with six Ru centers and one Rh center was more than seven times more efficient, cycling 300 times to produce hydrogen for 10 hours. This great disparity in efficiency and stability was puzzling because the supramolecules contain very similar components.
Manbeck joined Brookhaven in 2013 and has since carried out a series of experiments with coauthor Etsuko Fujita, leader of the artificial photosynthesis group, to understand the fundamental causes for the difference in performance.
"The ability to form the charge-separated state is a partial indicator of whether a supramolecule will be a good photocatalyst, but realizing efficient charge separation requires fine-tuning the energetics of each component," said Fujita. "To promote catalysis, the Rh catalyst must be low enough in energy to accept the electrons from the Ru light absorbers when the absorbers are exposed to light."
Through cyclic voltammetry, an electrochemical technique that shows the energy levels within a molecule, the scientists found that the Rh catalyst of the heptametallic system is slightly more electron-poor and thus more receptive to receiving electrons than its counterpart in the tetrametallic system. This result suggested that the charge transfer was favorable in the heptametallic but not the tetrametallic system.
They verified their hypothesis with a time-resolved technique called nanosecond transient absorption spectroscopy, in which a molecule is promoted to an excited state by an intense laser pulse and the decay of the excited state is measured over time. The resulting spectra revealed the presence of a Ru-to-Rh charge transfer in the heptametallic system only.
"The data not only confirmed our hypothesis but also revealed that the excited-state charge separation occurs much more rapidly than we had imagined," said Manbeck. "In fact, the charge migration happens faster than the time resolution of our instrument, and probably involves short-lived, high-energy excited states." The researchers plan to seek a collaborator with faster instrumentation who can measure the exact rate of charge separation to help clarify the mechanism.
In a follow-up experiment, the scientists performed the transient absorption measurement under photocatalytic operating conditions, with a reagent used as the ultimate source of electrons to produce hydrogen (a scalable artificial photosynthesis of hydrogen fuel from water would require replacing the reagent with electrons released during water oxidation). The excited state generated by the laser pulse rapidly accepted an electron from the reagent. They discovered that the added electron resides on Rh in the heptametallic system only, further supporting the charge migration to Rh predicted by cyclic voltammetry.
"The high photocatalytic turnover of the heptametallic system and the principles governing charge separation that were uncovered in this work encourage further studies using multiple light-harvesting units linked to single catalytic sites," said Manbeck.
This research is supported by DOE's Office of Science.
####
About Brookhaven National Laboratory
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov .
For more information, please click here
Contacts:
Ariana Tantillo, (631) 344-2347,
or Peter Genzer, (631) 344-3174,
Copyright © Brookhaven National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Tools
![]() Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Solar/Photovoltaic
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() Charged “molecular beasts” the basis for new compounds: Researchers at Leipzig University use “aggressive” fragments of molecular ions for chemical synthesis November 3rd, 2023
Charged “molecular beasts” the basis for new compounds: Researchers at Leipzig University use “aggressive” fragments of molecular ions for chemical synthesis November 3rd, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








