Home > Press > The Hydrogen-Fuel cell will revolutionize the economy of the world: New non-platinum and nanosized catalyst for polymer electrolyte fuel cell
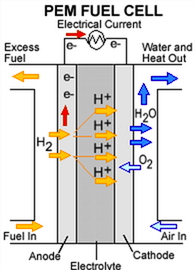 |
| Hydrogen PEMFC showing the anode catalyst layer where hydrogen gas is oxidized to hydrogen ions (protons), the cathode catalyst layer where oxygen reduction reaction occurs producing water and the electrolyte ( also called membrane or polymer electrolyte) where only protons migrate from the anode to the cathode to react with oxygen. |
Abstract:
Canadelectrochim have discovered a new non-platinum and nano-sized catalyst for the fuel cell based on Mother Nature which mimics the plant leaf.
The Hydrogen-Fuel cell will revolutionize the economy of the world: New non-platinum and nanosized catalyst for polymer electrolyte fuel cell
Calgary, Canada | Posted on June 29th, 2015PEMFC as an optimal solution for the future energy economy
Polymer electrolyte membrane or proton exchange membrane fuel cell (PEMFC), where chemical energy is directly converted to electrical energy, provides a highly efficient alternative to a standard internal combustion engine. High power density, clean emissions (water), low temperature operation, rapid start-up and shutdown, and ability to use fuels from renewable sources are several reason why fuel cells such as PEMFC have attracted attention for large market applications, such as transportation. With these unique features, PEMFC will revolutionize the future energy economy.
Modern applications for PEMFC
PEMFC will indirectly make water our future fuel. Hydrogen and oxygen generated by splitting water using photosynthesis can be used as a fuel for PEMFC. PEMFC are leading candidates to power the space shuttle and other mobile applications even down to mobile phones, however, there are still some important issues that must be resolved in order for PEMFC to be commercially competitive. It is known that splitting a hydrogen molecule at the anode of fuel cell using platinum is relatively easy. Unfortunately however, splitting the oxygen molecule at the cathode of fuel cell (oxygen reduction reaction (, ORR)) is more difficult and this causes significant polarization losses (lowers efficiency of the fuel cell). An appropriate catalyst for this process has not been discovered and as of yet platinum is the best option.
Platinum as the best element for use of PEMFC
Platinum is by far the most effective element used for PEMFC and nearly all current PEMFC uses platinum particles on porous carbon supports to catalyze both hydrogen oxidation and oxygen reduction .However, due to their high cost, current Pt/Carbon are not feasible for commercialization. The U.S. Department of Energy estimates that a platinum-based catalyst will need to use roughly four times less platinum than is used in current PEMFC designs in order to represent a realistic alternative to internal combustion engines (1, 2). Fuel cells generate electricity by combining hydrogen gas with oxygen to produce water (figure 1). Although that sounds perfectly clean and green, that hydrogen is typically generated by "reforming" fuels such as natural gas, gasoline, or ethanol, which invariably introduces carbon monoxide into the hydrogen gas. If even minute quantities of carbon monoxide are present in that gas, it can poison the platinum catalysts that are important to driving the fuel cell. (In the heart of a fuel cell, CO binds tightly to platinum and prevents it from grabbing hydrogen, the first step in the reaction.) However, Hydrogen produced from water splitting by photosynthesis is very clean and has zero carbon monoxide. An electrolyte or membrane is used to separate oxygen gas at the cathode region from hydrogen gas in the anodic region, while ions can still migrate from the anode to the cathode. The electrolyte plays a key role. It must permit only the appropriate ions to pass between the anode and cathode. If free electrons or other substances could travel through the electrolyte, they would short circuit the current in the fuel cell and fuel cell degradation occurs.
Advancements in the electrolyte system of PEMFC
The commercial development of a special electrolyte (single ion conducting polymer electrolyte) changed the field of electrochemical devices in a significant way. Electrochemists have spent many years in a continuing search for newer, more highly conducting (ions and not electrons) and a more electrochemically stable electrolyte system. With the development of a single ion (for example only hydrogen ions in PEMFC) conducting polymer, electrochemists have the ability to choose from a variety of polymers with both high conductivity for a given ion of interest (off course hydrogen ions in PEMFC) as well as excellent stability and process-ability allowing the design of electrochemical devices (such as PEMFC) in their most ideal format (3). The broad class of electrolyte (electrolyte is a polymer and so it is called polymer electrolyte) to which Nafion (discovered by DuPont company) belong has application in a number of area of commercial importance, not limited to PEMFC. When the PEMFC reaches its eventual position as the major power generation system in a broad-based application such as automotive propulsion, these Nafion electrolytes will reach a scale of production far exceeding the current levels. This change will bring about significant challenges for companies who manufacture the electrolyte but also offer tremendous opportunity. It is likely that these future ubiquitous electrolytes for PEMFC will look different, produce much less pollution during manufacturing and cost much less than the electrolyte that dominate the commercial landscape today.
Optimizing PEMFC and positive environmental impacts
Future PEMFC will have to use low grade (inexpensive) hydrogen gas, which contains impurities (e.g. carbon monoxide) that poison precious metal catalysts (e.g. platinum) only at low temperatures (less than 120 ̊ C) and reduces the PEMFC efficiency and power output. In addition to mitigating catalyst poisoning there are a number of advantages to operating the PEMFC at higher temperature and lower humidity, such as increasing catalytic activity, reducing cathode flooding and eliminating the need for external humidification equipment. However, at higher temperatures, current electrolyte that is used in PEMFC dehydrate (becomes very dry), reducing ionic conductivity ( no hydrogen ions would be able to migrate) and overall cell performance. This is because optimal operation for current PEMFC is 80 °C and 100 % relative humidity because Nafion is used as an electrolyte in these PEMFC. Thus there is a worldwide effort currently underway to find suitable alternatives to Nafion that might allow higher temperature operation and be more cost effective. In the direction of operating the fuel cell using a cost effective and non-platinum based catalyst, is the work of Canadelectrochim, based on Mother Nature idea. Mother Nature can build very efficient catalysts.
Environmental mechanisms sourced from Canadelectrochim
Canadelectrochim is a small Research and Development company located in Calgary, Alberta had presented a mechanism for the fuel cell reaction based on Mother Nature which mimics the plant leaf. This research work was presented in the 2011 and 2012 IMRC meeting in Cancun, Mexico. This work was based on the idea that rain droplets are known to roll or bounce on the surface of plant leaves, removing dust particles and contamination. This self- cleansing action is due to the presence of a special surface layer which is composed of microscale poles. Each of these microscale poles is covered with billions of nanoscale poles. In physics it is called “Heterogeneous superhydrophobic surface”. Based on the idea that has been called hierarchical roughness, a mechanism for the oxygen reduction reaction can be easily explained. Based on this mechanism the reaction of the fuel cell does not require platinum.
References
[1] Hydrogen, Fuel Cells & Infrastructure Technologies Program Multi-Year Research, Development and Demonstration Plan, U.S. Department of Energy, October 2007.
[2] Hydrogen, Fuel Cells & Infrastructure Technologies Program Multi-Year Research, Development and Demonstration Plan, U.S. Department of Energy, October 2007.
[3] M. Doyle and G. Rajendran, Chapter 10, Handbook of fuel cell, edited by W. Vielstich, Volume 3: Fuel cell technology and Applications, John Wiley Ltd. (2003)
####
For more information, please click here
Contacts:
M. Reda
Phone: 4034673552
Copyright © Canadelectrochim
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Artificial Intelligence
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() New chip opens door to AI computing at light speed February 16th, 2024
New chip opens door to AI computing at light speed February 16th, 2024
![]() HKUST researchers develop new integration technique for efficient coupling of III-V and silicon February 16th, 2024
HKUST researchers develop new integration technique for efficient coupling of III-V and silicon February 16th, 2024
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








