Home > Press > Engineers teach old chemical new tricks to make cleaner fuels, fertilizers: Researchers from Denmark and Stanford show how to produce industrial quantities of hydrogen without emitting carbon into the atmosphere
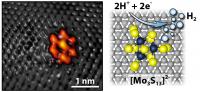 |
| On the left, a scanning tunneling microscope image captures the bright shape of the moly sulfide nanocluster on a graphite surface. The grey spots are carbon atoms. Together the moly sulfide and graphite make the electrode. The diagram on the right shows how two positive hydrogen ions gain electrons through a chemical reaction at the moly sulfide nanocluster to form pure molecular hydrogen.
Credit: Jakob Kibsgaard |
Abstract:
University researchers from two continents have engineered an efficient and environmentally friendly catalyst for the production of molecular hydrogen (H2), a compound used extensively in modern industry to manufacture fertilizer and refine crude oil into gasoline.
Engineers teach old chemical new tricks to make cleaner fuels, fertilizers: Researchers from Denmark and Stanford show how to produce industrial quantities of hydrogen without emitting carbon into the atmosphere
Stanford, CA | Posted on January 27th, 2014Although hydrogen is abundant element, it is generally not found as the pure gas H2but is generally bound to oxygen in water (H2O) or to carbon in methane (CH4), the primary component in natural gas. At present, industrial hydrogen is produced from natural gas using a process that consumes a great deal of energy while also releasing carbon into the atmosphere, thus contributing to global carbon emissions.
In an article published today (Jan. 26, 1300 EST) in Nature Chemistry, nanotechnology experts from Stanford Engineering and from Denmark's Aarhus University explain how to liberate hydrogen from water on an industrial scale by using electrolysis .
In electrolysis, electrical current flows through a metallic electrode immersed in water. This electron flow induces a chemical reaction that breaks the bonds between hydrogen and oxygen atoms. The electrode serves as a catalyst, a material that can spur one reaction after another without ever being used up. Platinum is the best catalyst for electrolysis. If cost were no object, platinum might be used to produce hydrogen from water today.
But money matters. The world consumes about 55 billion kilograms of hydrogen per year. It now costs about $1 to $2 per kilogram to produce hydrogen from methane. So any competing process, even if it's greener, must hit that production cost, which rules out electrolysis based on platinum.
In their Nature Chemistry paper, the researchers describe how they re-engineered the atomic structure of a cheap and common industrial material to make it nearly as efficient at electrolysis as platinum - a finding that has the potential to revolutionize industrial hydrogen production.
The project was conceived by Jakob Kibsgaard, a post-doctoral researcher with Thomas Jaramillo, an assistant professor of chemical engineering at Stanford. Kibsgaard started this project while working with Flemming Besenbacher, a professor at the Interdisciplinary Nanoscience Center (iNANO) at Aarhus.
Subhead: Meet Moly Sulfide
Since World War II petroleum engineers have used molybdenum sulfide - moly sulfide for short - to help refine oil.
Until now, however, this chemical was not considered a good catalyst for making moly sulfide to produce hydrogen from water through electrolysis. Eventually scientists and engineers came to understand why: the most commonly used moly sulfide materials had an unsuitable arrangement of atoms at their surface.
Typically, each sulfur atom on the surface of a moly sulfide crystal is bound to three molybdenum atoms underneath. For complex reasons involving the atomic bonding properties of hydrogen, that configuration isn't conducive to electrolysis.
In 2004, Stanford chemical engineering professor Jens Norskov, then at the Technical University of Denmark, made an important discovery. Around the edges of the crystal, some sulfur atoms are bound to just two molybdenum atoms. At these edge sites, which are characterized by double rather than triple bonds, moly sulfide was much more effective at forming H2.
Armed with that knowledge, Kibsgaard found a 30-year-old recipe for making a form of moly sulfide with lots of these double-bonded sulfurs at the edge.
Using simple chemistry, he synthesized nanoclusters of this special moly sulfide. He deposited these nanoclusters onto a sheet of graphite, a material that conducts electricity. Together the graphite and moly sulfide formed a cheap electrode. It was meant to be a substitute for platinum, the ideal but expensive catalyst for electrolysis.
The question then became: could this composite electrode efficiently spur the chemical reaction that rearranges hydrogen and oxygen atoms in water?
As Jaramillo put it: "Chemistry is all about where electrons want to go, and catalysis is about getting those electrons to move to make and break chemical bonds."
Subhead: The acid test
So the experimenters put their system to the acid test -- literally.
They immersed their composite electrode into water that was slightly acidified, meaning it contained positively charged hydrogen ions. These positive ions were attracted to the moly sulfide clusters. Their double-bonded shape gave them just the right atomic characteristic to pass electrons from the graphite conductor up to the positive ions. This electron transfer turned the positive ions into neutral molecular hydrogen, which bubbled up and away as a gas.
Most importantly, the experimenters found that their cheap, moly sulfide catalyst had the potential to liberate hydrogen from water on something approaching the efficiency of a system based on prohibitively expensive platinum.
Subhead: Yes, but does it scale?
But in chemical engineering, success in a beaker is only the beginning.
The larger questions were: could this technology scale to the 55 billion kilograms per year global demand for hydrogen, and at what finished cost per kilogram?
Last year, Jaramillo and a dozen co-authors studied four factory-scale production schemes in an article for The Royal Society of Chemistry's journal of Energy and Environmental Science.
They concluded that it could be feasible to produce hydrogen in factory-scale electrolysis facilities at costs ranging from $1.60 and $10.40 per kilogram - competitive at the low end with current practices based on methane -- though some of their assumptions were based on new plant designs and materials.
"There are many pieces of the puzzle still needed to make this work, and much effort ahead to realize them," Jaramillo said. "However, we can get huge returns by moving from carbon-intensive resources to renewable, sustainable technologies to produce the chemicals we need for food and energy."
####
For more information, please click here
Contacts:
Tom Abate
650-736-2245
Copyright © Stanford School of Engineering
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Food/Agriculture/Supplements
![]() Silver nanoparticles: guaranteeing antimicrobial safe-tea November 17th, 2023
Silver nanoparticles: guaranteeing antimicrobial safe-tea November 17th, 2023
![]() Night-time radiative warming using the atmosphere November 17th, 2023
Night-time radiative warming using the atmosphere November 17th, 2023
![]() DGIST and New Life Group launched a research project on "Functional beauty and health products using the latest nanotechnology" May 12th, 2023
DGIST and New Life Group launched a research project on "Functional beauty and health products using the latest nanotechnology" May 12th, 2023
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Industrial
![]() Boron nitride nanotube fibers get real: Rice lab creates first heat-tolerant, stable fibers from wet-spinning process June 24th, 2022
Boron nitride nanotube fibers get real: Rice lab creates first heat-tolerant, stable fibers from wet-spinning process June 24th, 2022
![]() Nanotubes: a promising solution for advanced rubber cables with 60% less conductive filler June 1st, 2022
Nanotubes: a promising solution for advanced rubber cables with 60% less conductive filler June 1st, 2022
![]() Protective equipment with graphene nanotubes meets the strictest ESD safety standards March 25th, 2022
Protective equipment with graphene nanotubes meets the strictest ESD safety standards March 25th, 2022
![]() OCSiAl receives the green light for Luxembourg graphene nanotube facility project to power the next generation of electric vehicles in Europe March 4th, 2022
OCSiAl receives the green light for Luxembourg graphene nanotube facility project to power the next generation of electric vehicles in Europe March 4th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








