Home > Press > Researchers discover a new protein fold with a transport tunnel: Biochemists from Bielefeld, Toronto, Boston, and Kiel publish study in Nature
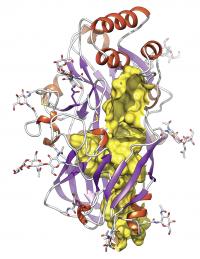 |
| The Bielefeld chemist Michael Schwake and his colleagues have discovered a new protein fold. At its head (the red helices), this protein can bind enzymes and viruses. The tunnel in the protein structure is colored yellow.
Credit: Illustration: Nature |
Abstract:
The protein LIMP-2 is vital for both humans and animals. If it is absent - due, for example, to a hereditary disease - substances of an unknown nature, probably lipids, accumulate in the organism. Up to now, scientists were unsure what the protein looks like and how exactly it functions. Privatdozent [senior lecturer] Dr. Michael Schwake from the Faculty of Chemistry at Bielefeld University (Germany) is doing research on the protein - and thereby preparing the way for future therapies. Together with colleagues in Kiel, Toronto, and Boston, he has now discovered that the protein LIMP 2 possesses a novel protein fold together with a nanomicroscopically small transport tunnel. The researchers have published their findings on Sunday (27 October) in the globally renowned scientific journal Nature.
Researchers discover a new protein fold with a transport tunnel: Biochemists from Bielefeld, Toronto, Boston, and Kiel publish study in Nature
Bielefeld, Germany | Posted on October 28th, 2013Proteins are composed of amino acids. Although these are lined up as if along a string, they produce a twisted three-dimensional structure of helices and sheets. It is only this pleating that enables them to influence biological cells. 'We are decoding the structure and function of proteins in order to find out how biochemical processes within them take place,' says Schwake.
To study LIMP-2, Schwake's colleagues from the Canadian University of Toronto have crystallized the protein. Then they can use X-ray diffraction analysis to ascertain its crystalline structure. 'When analysing the images, we detected a protein fold that has not been described in any other protein up to now,' says Schwake.
LIMP-2 is present in every cell of the human body. It is found mostly in the lysosomes of the cells where it ensures that a specific enzyme reaches them. Lysosomes are the 'stomachs' of the cells and they break down harmful and unusable substances. A specific enzyme called beta-glucocerebrosidase is responsible for breaking down lipids. If this enzyme is defect or does not reach the lysosomes, these lipids will accumulate. Biochemists suspect that this is what causes Gaucher's disease that leads to an enlarged liver and spleen.
Schwake's studies confirm how LIMP-2 transports this enzyme. The protein has a 'head' consisting of several helices on which the enzyme docks. 'We also managed to show that the protein is equipped with a tunnel through which it transports substances through membranes,' Schwake reports. The biochemists have determined that it is highly probable that this channel is used to transport lipids away from the lysosome. 'We determined that by comparing the structure of LIMP-2 with that of related proteins,' says Schwake. Two of these proteins are known to bind and transport lipids. The comparison suggests that LIMP-2 must possess the same ability.
As a biochemist, it is not Schwake's job to develop a therapy - his interest is in basic research, that is, in finding out how the proteins work in the cells. 'Our findings could be used to develop substances to cure diseases,' he explains. 'Through our research, we now how ligands bind to the head and lipids are transported through the tunnel. One way to prevent this would be to deliberately disrupt the binding at these locations,' says Schwake.
Since February 2013, PD Dr. Michael Schwake has been running a research team in the Biochemistry III Research Group at Bielefeld University's Faculty of Chemistry headed by Professor Dr. Gabriele Fischer von Mollard. Before this, he was a researcher at the Institute of Biochemistry at Kiel University and at Stanford University (California). Schwake took his doctorate in 2001 at the Center for Molecular Neurobiology Hamburg and his post-doctoral habilitation in 2007 at Kiel University. For the study on LIMP-2, he worked with Professor Dr. Paul Saftig from the Institute of Biochemistry at Kiel University. He also cooperated with researchers at the Dana-Farber Cancer Institute in Boston (USA), the University of Toronto (Canada), the Li Ka Shing Knowledge Institute, and the SickKids Research Institute, both in Toronto.
####
For more information, please click here
Contacts:
Dr. Michael Schwake
49-521-106-2091
Copyright © University of Bielefeld
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Imaging
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
![]() Observation of left and right at nanoscale with optical force October 6th, 2023
Observation of left and right at nanoscale with optical force October 6th, 2023
Nanomedicine
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
![]() Researchers develop artificial building blocks of life March 8th, 2024
Researchers develop artificial building blocks of life March 8th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Nanobiotechnology
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
![]() Researchers develop artificial building blocks of life March 8th, 2024
Researchers develop artificial building blocks of life March 8th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








