Home > Press > Size matters in nanocrystals' ability to adsorb/release gases
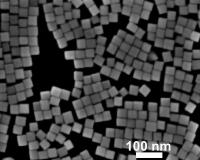 |
| These are palladium nanocrystals.
Credit: Bardhan Laboratory |
Abstract:
More efficient catalytic converters on autos, improved batteries and more sensitive gas sensors are some of the potential benefits of a new system that can directly measure the manner in which nanocrystals adsorb and release hydrogen and other gases.
Size matters in nanocrystals' ability to adsorb/release gases
Nashville, TN | Posted on August 8th, 2013The technique, which was developed by Vanderbilt University Assistant Professor of Chemical and Biomolecular Engineering Rizia Bardhan, is described in a paper published online Aug. 4 by the journal Nature Materials.
In the last 30 years, there has been a tremendous amount of research studying nanocrystals - tiny crystals sized between one to 100 nanometers in size (a nanometer is to an inch what an inch is to 400 miles) - because of the expectation that they have unique physical and chemical properties that can be used in a broad range of applications.
One class of applications depends on nanocrystals' ability to grab specific molecules and particles out the air, hold on to them and then release them: a process called adsorption and desorption. Progress in this area has been hindered by limitations in existing methods for measuring the physical and chemical changes that take place in individual nanocrystals during the process. As a result, advances have been achieved by trial-and-error and have been limited to engineered samples and specific geometries.
"Our technique is simple, direct and uses off-the shelf instruments so other researchers should have no difficulty using it," said Bardhan. Collaborators in the development were Vanderbilt Assistant Professor of Mechanical Engineering Cary Pint, Ali Javey from the University of California, Berkeley and Lester Hedges, Stephen Whitelam and Jeffrey Urban from the Lawrence Berkeley National Laboratory.
The method is based on a standard procedure called fluorescence spectroscopy. A laser beam is focused on the target nanocrystals, causing them to fluoresce. As the nanocrystals adsorb the gas molecules, the strength of their fluorescent dims and as they release the gas molecules, it recovers.
"The fluorescence effect is very subtle and very sensitive to differences in nanocrystal size," she explained. "To see it you must use nanocrystals that are uniform in size." That is one reason why the effect wasn't observed before: Fabrication techniques such as ball milling and other wet-chemical approaches that have been widely used produce nanocrystals in a range of different sizes. These differences are enough to mask the effect.
To test their technique, the researchers studied hydrogen gas sensing with nanocrystals made out of palladium. They choose palladium because it is very stable and it readily releases adsorbed hydrogen. They used hydrogen because of the interest in using it as a replacement for gasoline. One of the major technical obstacles to this scenario is developing a safe and cost-effective storage method. A nanocrystal-based metal hydride system is one of the promising approaches under development.
The measurements they made revealed that the size of the nanocrystals have a much stronger effect on the rate that the material can adsorb and release hydrogen and the amount of hydrogen that the material can absorb than previously expected - all key properties for a hydrogen storage system. The smaller the particle size, the faster the material can absorb the gas, the more gas it can absorb and faster it can release it.
"In the past, people thought that the size effect was limited to sizes less than 15 to 20 nanometers, but we found that it extends up to 100 nanometers," said Bardhan.
The researchers also determined that the adsorption/desorption rate was determined by just three factors: pressure, temperature and nanocrystal size. They did not find that additional factors such as defects and strain had a significant effect as previously suggested. Based on this new information, they created a simple computer simulation that can predict the adsorption/desorption rates of various types and size ranges of nanocrystals with a variety of different gases.
"This makes it possible to optimize a wide range of nanocrystal applications, including hydrogen storage systems, catalytic converters, batteries, fuel cells and supercapacitors," Bardhan said.
The research was funded by Department of Energy grants KC0202020 and AC02-05CH11231.
####
For more information, please click here
Contacts:
David F. Salisbury
615-343-6803
Copyright © Vanderbilt University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Automotive/Transportation
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








