Home > Press > Scientists Spy on Lithium Ions
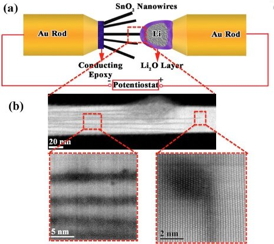 |
| Above (a), the nanobattery setup inside AC-STEM. Below (b), atomic resolution imaging of the front line of lithium ions entering a SnO2 nanowire. The images show the parallel Li-ion channels and the formation of dislocations at the tip of the channels. |
Abstract:
Lithium ion batteries are at the energetic heart of almost all things tech, from cell phones to tablets to electric vehicles. That's because they are a proven technology, light, long-lasting and powerful. But they aren't perfect.
Scientists Spy on Lithium Ions
Houghton, MI | Posted on July 8th, 2013"You might get seven or eight hours out of your iPhone on one charge, maybe a day," says Reza Shahbazian-Yassar, an associate professor of mechanical engineering at Michigan Technological University. "This is not enough for many of us. A fully electric car, like the Nissan Leaf, can go up to 100 miles on a single charge. To appeal to a mass market, it should be about 300 miles. We want to increase the power of these systems."
To wring more power out of lithium ion batteries, scientists are experimenting with different materials and designs. However, the important action in a battery occurs at the atomic level, and it's been virtually impossible to find out exactly what's happening at such a scale. Now, Yassar has developed a device that allows researchers to eavesdrop on individual lithium ions—and potentially develop the next generation of batteries.
Batteries are pretty simple. They have three major components: an anode, a cathode and electrolyte between the two. In lithium batteries, lithium ions travel back and forth between the anode and cathode as the battery discharges and is charged up again. The anodes of lithium-ion batteries are usually made of graphite, but scientists are testing other materials to see if they can last longer.
"As soon as lithium moves into an electrode, it stresses the material, eventually resulting in failure," said Yassar. "That's why many of these materials may be able to hold lots of lithium, but they end up breaking down quickly.
"If we were able to observe these changes in the host electrode, particularly at the very early stage of charging, we could come up with strategies to fix that problem."
Ten years ago, observing light elements such as lithium or hydrogen at the atomic level would have been out of the question. Now, however, it's possible to see light atoms with an aberration corrected scanning transmission electron microscope (AC-STEM). Yassar's team was able to use one at the University of Illinois at Chicago, where he is a visiting associate professor.
To determine how the host electrode changes as lithium ions enter it, the team built a nano-battery within the AC-STEM microscope using a promising new electrode material, tin oxide, or SnO2. Then, they watched it charge.
"We wanted to monitor the changes in the tin oxide at the very frontier of lithium-ion movement within the SnO2 electrode, and we did," Yassar said. "We were able to observe how the individual lithium ions enter the electrode."
The lithium ions moved along specific channels as they flowed into the tin oxide crystals instead of randomly walking into the host atoms. Based on that data, the researchers were able to calculate the strain the ions were placing on the electrodes.
The discovery has prompted inquiries from industries and national labs interested in using his atomic-resolution capability in their own battery-development work.
"It's very exciting," Yassar said. "There are so many options for electrodes, and now we have this new tool that can tell us exactly what's happening with them. Before, we couldn't see what was going on; we were just guessing."
An article on the research, "Atomic Scale Observation of Lithiation Reaction Front in Nanoscale SnO2 Materials," was published online June 3 in ACS Nano. In addition to Yassar, the coauthors are mechanical engineering graduate student Hasti Asayesh-Ardakani and research associate Anmin Nie of Michigan Tech; Li-Yong Gan, Yingchun Cheng and Udo Schwingeschlogl of King Abdullah University of Science and Technology, Saudi Arabia; Qianquin Li, Cezhou Dong and Tao Wang of Zhejiang University, China; and Farzad Mashayek and Robert Klie of the University of Illinois at Chicago.
####
About Michigan Technological University
Michigan Technological University (www.mtu.edu) is a leading public research university developing new technologies and preparing students to create the future for a prosperous and sustainable world. Michigan Tech offers more than 130 undergraduate and graduate degree programs in engineering; forest resources; computing; technology; business; economics; natural, physical and environmental sciences; arts; humanities; and social sciences. - See more at: http://www.mtu.edu/news/stories/2013/july/story92744.html#sthash.cMSWQZl7.dpuf
For more information, please click here
Contacts:
Marcia Goodrich
906-487-2343
Copyright © Michigan Technological University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Imaging
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
![]() Observation of left and right at nanoscale with optical force October 6th, 2023
Observation of left and right at nanoscale with optical force October 6th, 2023
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Tools
![]() Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
Automotive/Transportation
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








