Home > Press > Nanobiotix announces the selection of its second NanoXray product, NBTX-IV and a collaboration with the National Cancer Institute for development: Characterization studies to be undertaken by the US National Cancer Institute’s Nanotechnology Characterization Laboratory
 |
Abstract:
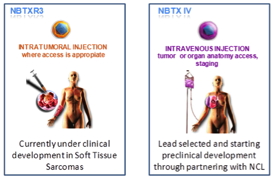
NANOBIOTIX (Euronext: NANO), a clinical-stage nanomedicine company pioneering novel approaches for the local treatment of cancer, announces the selection of a new product for development, NBTX-IV. It is the second product of the NanoXray pipeline which is designed for systemic administration (intravenous injection). This product has been selected by the National Cancer Institute's (NCI) Nanotechnology Characterization Laboratory (NCL) for characterization on the basis of its potential to impact cancer treatment.
Nanobiotix announces the selection of its second NanoXray product, NBTX-IV and a collaboration with the National Cancer Institute for development: Characterization studies to be undertaken by the US National Cancer Institute’s Nanotechnology Characterization Laboratory
Paris, France | Posted on June 26th, 2013NBTX-IV is based on Nanobiotix's proprietary NanoXray platform. It is designed to be administered intravenously to target deep-seated tumors and lymph nodes which may have been invaded locally by cancer cells. The product aims to enhance radiotherapy energy to destroy cancer cells and reduce the subsequent escape of malignant cells localized in neighboring tissues cells or lymph nodes. Target indications include lung carcinoma, pancreatic cancer or brain metastases.
As part of the collaboration with NCI, the NCL will perform pre-clinical characterization of NBTX-IV that will support Nanobiotix's filing of an Investigational New Drug (IND) with the FDA. In parallel, Nanobiotix will conduct additional preclinical testing to provide a complete dossier for submission.
The NCL was established to investigate the use of nanoparticulate material for the advancement of cancer research and to accelerate the development of promising and safe nanotechnology-derived cancer therapeutics. It provides preclinical testing and consultation services on a competitive basis to developers, like Nanobiotix, and is working in concert with other US agencies such as the U.S. Food and Drug Administration (FDA) to accelerate the transition of basic nanomedicine research into clinical applications.
"The selection of a second NanoXray product for development coupled with the characterization being undertaken by the NCL is an important strategic step for Nanobiotix," said Laurent Levy, CEO of Nanobiotix. "This collaboration with the NCL will hopefully accelerate the pre-clinical development of NBTX-IV, opening the pathway for new clinical indications in addition to the one covered by our first product. The results of the characterization are not only important for our future IND submission, but also raise our profile in the US market."
####
About Nanobiotix
Nanobiotix (Euronext: NANO / ISIN: FR0011341205) is a clinical-stage nanomedicine company pioneering novel approaches for the local treatment of cancer. The Company’s first-in-class, proprietary technology, NanoXray, enhances radiotherapy energy to provide a new, more efficient treatment for cancer patients. NanoXray products are compatible with current radiotherapy treatments and are meant to treat a wide variety of cancers via multiple routes of administration. Nanobiotix’s lead product NBTXR3, based on NanoXray, is currently under clinical development for soft tissue sarcoma. The Company has partnered with PharmaEngine for clinical development and commercialization of NBTXR3 in Asia. The Company is based in Paris, France.
About NANOXRAY
Nanobiotix’s first-in-class, proprietary technology called NanoXray is at the forefront of a new era of nanomedicine, where nanoparticles are not just a vehicle for targeted drug delivery, but have become the principal active element. The NanoXray technology is based on the physical properties of hafnium-oxide nanoparticles and is used to enhance the efficacy of radiotherapy treatment for a variety of cancer indications.
Nanoparticles are designed to enter tumor cells and, upon activation by a standard dose of radiation, they emit large amounts of electrons resulting in the generation of free radicals that destroy cancer cells (the same mode of action than radiotherapy but largely amplified). Nanoparticle-enhanced radiotherapy therefore amplifies the lethal dose of energy locally within the tumor without changing the effect of the dose passing through surrounding healthy tissues.
By changing the coating of the nanoparticles, Nanobiotix is developing three different products that can be administered either by direct injection into the tumor (NBTXR3), intravenous injection (NBTX-IV) or topical application to fill tumor cavities after surgery (NBTX-TOPO). The product applied will depend on type of tumor and the patient’s specific clinical needs. NanoXray products are classified as a medical device in Europe and as a drug in the US. They are compatible with current radiotherapy methods with respect to equipment and protocols, as well as with older radiotherapy equipment or any radiation-based therapy.
Disclaimer
This press release contains certain forward-looking statements concerning Nanobiotix and its business. Such forward-looking statements are based on assumptions that Nanobiotix considers to be reasonable. However, there can be no assurance that the estimates contained in such forward-looking statements will be verified, which estimates are subject to numerous risks including the risks set forth in the 2012 financial annual report of Nanobiotix (a copy of which is available on www.nanobiotix.com) and to the development of economic conditions, financial markets and the markets in which Nanobiotix operates. The forward-looking statements contained in this press release are also subject to risks not yet known to Nanobiotix or not currently considered material by Nanobiotix. The occurrence of all or part of such risks could cause actual results, financial conditions, performance or achievements of Nanobiotix to be materially different from such forward-looking statements.
This press release and the information that it contains do not constitute an offer to sell or subscribe for, or a solicitation of an offer to purchase or subscribe for, Nanobiotix shares in any country.
For more information, please click here
Contacts:
Nanobiotix
Laurent Levy
CEO
+33 (0)1 40 26 07 55
Yucatan
Media relations (France)
Annie-Florence Loyer/
Nadège Le Lezec
+33 (0)1 53 63 27 27
+33 (0)6 88 20 35 59
NewCap.
Financial communication
and investors relations
Louis-Victor Delouvrier /
Emmanuel Huynh
+33 (0)1 44 71 98 53
College Hill
Media relations (Outside France)
Melanie Toyne Sewell / Donia Al Saffar
+44 (0) 207 457 2020
Copyright © Nanobiotix
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Nanomedicine
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
![]() Researchers develop artificial building blocks of life March 8th, 2024
Researchers develop artificial building blocks of life March 8th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Alliances/Trade associations/Partnerships/Distributorships
![]() Chicago Quantum Exchange welcomes six new partners highlighting quantum technology solutions, from Chicago and beyond September 23rd, 2022
Chicago Quantum Exchange welcomes six new partners highlighting quantum technology solutions, from Chicago and beyond September 23rd, 2022
![]() University of Illinois Chicago joins Brookhaven Lab's Quantum Center June 10th, 2022
University of Illinois Chicago joins Brookhaven Lab's Quantum Center June 10th, 2022
Research partnerships
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() 'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








