Home > Press > Efficient and inexpensive: Researchers develop catalyst material for fuel cells: Platinum-nickel nano-octahedra save 90 percent platinum
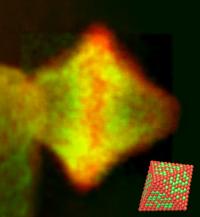 |
| Electron micrograph and atomistic model (bottom right) of a highly oxygen-activating platinum-nickel catalyst particle. Its diameter is approximately ten thousand times smaller than the diameter of a human hair. Red spheres represent platinum atoms and green spheres represent nickel atoms. One of the properties of such octahedra is that most surface atoms have the same geometric arrangement. The micrograph was taken at the PICO microscope.
Credit: Source: Forschungszentrum Jülich/TU Berlin |
Abstract:
Efficient, robust and economic catalyst materials hold the key to achieving a breakthrough in fuel cell technology. Scientists from Jülich and Berlin have developed a material for converting hydrogen and oxygen to water using a tenth of the typical amount of platinum that was previously required. With the aid of state-of-the-art electron microscopy, the researchers discovered that the function of the nanometre-scale catalyst particles is decisively determined by their geometric shape and atomic structure. This discovery opens up new paths for further improving catalysts for energy conversion and storage. The results have been published in the current issue of the respected journal Nature Materials (DOI: 10.1038/nmat3668).
Efficient and inexpensive: Researchers develop catalyst material for fuel cells: Platinum-nickel nano-octahedra save 90 percent platinum
Jülich, Germany | Posted on June 17th, 2013Hydrogen-powered fuel cells are regarded as a clean alternative to conventional combustion engines, as, aside from electric energy, the only substance produced during operation is water. At present, the implementation of hydrogen fuel cells is being hindered by the high material costs of platinum. Large quantities of the expensive noble metal are still required for the electrodes in the fuel cells at which the chemical conversion processes take place. Without the catalytic effect of the platinum, it is not currently possible to achieve the necessary conversion rates.
As catalysis takes place at the surface of the platinum only, material can be saved and, simultaneously, the efficiency of the electrodes improved by using platinum nanoparticles, thus increasing the ratio of platinum surface to material required. Although the tiny particles are around ten thousand times smaller than the diameter of a human hair, the surface area of a kilogram of such particles is equivalent to that of several football fields.
Still more platinum can be saved by mixing it with other, less valuable metals, such as nickel or copper. Scientists from Forschungszentrum Jülich and Technische Universität Berlin have succeeded in developing efficient metallic catalyst particles for converting hydrogen and oxygen to water using only a tenth of the typical amount of platinum that was previously required.
The new catalyst consists not of the round nanoparticles that were previously in widespread use, but of octrahedral-shaped nanoparticles of a platinum-nickel alloy. The researchers discovered that the unique manner in which the platinum and nickel atoms arrange themselves on the surfaces of these particles serves to optimally accelerate the chemical reaction between hydrogen and oxygen to form water. Round or cubic particles, on the other hand, have different atomic arrangements at the surface and are therefore less effective catalysts for the chemical reaction, something which would have to be compensated by using increased amounts of noble metal.
The way in which the life-cycle of the catalysts depends on and can be optimized by their atomic composition was the subject of the research team's investigation, which made use of ultrahigh-resolution electron microscopy at the Ernst Ruska-Centre (ER-C), a facility of the Jülich Aachen Research Alliance. "A decisive factor for understanding the life-cycle of the catalysts was the observation that nickel and platinum atoms prefer not to be evenly distributed at the surface of the nano-octahedra," explains Dr. Marc Heggen from ER-C and the Peter Grünberg Institute at Forschungszentrum Jülich. "Although this is advantageous for reactivity, it limits lifetime."
To identify the location of each element with atomic precision, the researchers used a method in which the electron beam of one of the world's leading ultrahigh-resolution electron microscopes is finely focused, sent through the specimen and, by interactions with the specimen, loses part of its energy. Each element in the specimen can thus be identified like a fingerprint. Conventional electron microscopes are not capable of detecting such chemical signatures with atomic resolution.
"This pioneering experimental work provides direct evidence for the fact that the choice of the correct geometric shape for the catalyst particles is as important for optimizing their function as the choice of their composition and size," says Prof. Peter Strasser from Technische Universität Berlin. "This provides researchers with new possibilities for further improving functional materials, especially catalysts, for energy storage." The latest experiments from Strasser's research group indicate that substantial increases in efficiency may also be possible for the reaction splitting water to produce oxygen in electrolysers, for which the even more expensive noble metal iridium is used.
###
Original publication:
Compositional segregation in shaped Pt alloy nanoparticles and their structural behavior during electrocatalysis
C. Cui, L. Gan, M. Heggen, S. Rudi, P. Strasser
Nature Materials, published online: 16 June 2013; DOI: 10.1038/nmat3668
####
About Jülich, Germany
Forschungszentrum Jülich… ... pursues cutting-edge interdisciplinary research addressing pressing issues facing society today, above all the energy supply of the future. With its competence in materials science and simulation and its expertise in physics, nanotechnology and information technology, as well as in the biosciences and brain research, Jülich is developing the basis for the key technologies of tomorrow. Forschungszentrum Jülich helps to solve the grand challenges facing society in the fields of energy and the environment, health, and information technology. With almost 5000 employees, Jülich – a member of the Helmholtz Association – is one of the large interdisciplinary research centres in Europe.
For more information, please click here
Contacts:
Dr. Marc Heggen
Forschungszentrum Jülich
Microstructure Research (PGI-5)
tel: +49 2461 61-9479
Prof. Dr. Peter Strasser
Technische Universität Berlin
Department of Chemistry
tel: +49 30 314-29542
Press contact:
Angela Wenzik
science journalist
Forschungszentrum Jülich
tel: +49 2461 61-6048
Copyright © Jülich, Germany
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() Peter Grünberg Institute, Microstructure Research (PGI-5):
Peter Grünberg Institute, Microstructure Research (PGI-5):
![]() TU Berlin, Department of Chemistry:
TU Berlin, Department of Chemistry:
![]() High-performance microscopy at ER-C – how the PICO works:
High-performance microscopy at ER-C – how the PICO works:
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Imaging
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Tools
![]() Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Automotive/Transportation
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








