Home > Press > Thin films of nickel and iron oxides yield efficient solar water-splitting catalyst: Basic University of Oregon research shows promise in efforts to get hydrogen fuel from sunlight and water
 |
Abstract:
University of Oregon chemists say that ultra-thin films of nickel and iron oxides made through a solution synthesis process are promising catalysts to combine with semiconductors to make devices that capture sunlight and convert water into hydrogen and oxygen gases.
Thin films of nickel and iron oxides yield efficient solar water-splitting catalyst: Basic University of Oregon research shows promise in efforts to get hydrogen fuel from sunlight and water
Eugene, OR | Posted on March 20th, 2013Researchers in the Solar Materials and Electrochemistry Laboratory of Shannon Boettcher, professor of chemistry, studied the catalyst material and also developed a computer model for applying catalyst thin films in solar water-splitting devices as a tool to predict the effectiveness of a wide range of catalyst materials for solar-hydrogen production.
The project has resulted in two recent papers.
The first, detailed last September in the Journal of the American Chemical Society, showed that films of a nickel-iron mixed oxide with an atomic structure similar to naturally occurring minerals show the highest catalytic activity for forming oxygen from water, based on a side-by-side comparison of eight oxide-based materials targeted in various research efforts. The second paper, just published in the Journal of Physical Chemistry Letters, details the performance of the catalyst thin films when combined with semiconductor light absorbers, showing that the nickel-iron oxide catalyst was most effective with a film just 0.4 nanometers thick.
Boettcher's lab, located in the UO's Materials Science Institute, studies fundamental materials chemistry and physical concepts related to the conversion of solar photons (sunlight) into electrons and holes in semiconductors that can then be used to drive chemical processes such as splitting protons off water to make hydrogen and oxygen gases. Multiple labs across the country are seeking effective and economical ways of taking sunlight and directly producing hydrogen gas as an alternative sustainable fuel to replace fossil fuels.
"When you want to pull the protons off a water molecule to make hydrogen gas for fuel, you also have to take the leftover oxygen atoms and make oxygen gas out of them," Boettcher said. "It turns out that the slowest, hardest, most-energy-consuming step in the water-splitting process is actually the oxygen-making step. We've been studying catalysts for making oxygen. Specifically, we're seeking catalysts that reduce the amount of energy it takes in this step and that don't use expensive precious metals."
The iron-nickel oxides, he said, have higher catalytic activity than the precious-metal-based catalytic materials that have been thought to be the best for the job.
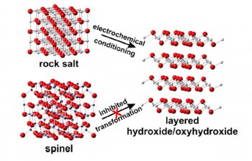
"What we found is that when we take nickel oxide films that start out as a crystalline material with the rock-salt structure like table salt, they absorb iron impurities and spontaneously convert into materials with a layered structure during the catalysis process," Boettcher said.
Lena Trotochaud, a doctoral student and lead author on both papers, studied this process and how the films can be combined with semiconductors. "The semiconductors absorb the light, generating electron-hole pairs which move onto the catalyst material and proceed to drive the water-splitting reaction, creating fuel," Boettcher said.
The computer modeling was used to understand how the amount of sunlight that the catalyst blocks from reaching the semiconductor can be minimized while simultaneously speeding up the reaction with water to form oxygen gas. This basic discovery remains a lab accomplishment for now, but it could advance to testing in a prototype device, Boettcher added.
"We're now looking at the fundamental reasons why these materials are good," Trotochaud said. "We are trying to understand how the catalyst works by focusing on the chemistry that is happening, and then also recognizing how that fits into a real system. Our research is fundamentally guiding how you would take these catalysts and incorporate them into something that is useful for everyone in society."
One such place the material could land in a prototype for testing is at the U.S. Department of Energy's Joint Center for Artificial Photosynthesis, an Energy Innovation Hub. The DOE supported Boettcher's research done in the second study through a Basic Sciences Energy grant (DE-FG02-12ER16323).
"This research holds great potential for the development of more efficient, more sustainable solar-fuel generation systems and other kinds of transformative energy technology," said Kimberly Andrews Espy, vice president for research and innovation and dean of the graduate school. "By seeking to advance carbon-neutral energy technology, Dr. Boettcher and his team are helping to establish Oregon as an intellectual and economic leader in fostering a sustainable future for our planet and its people."
The research reported in the first paper in JACS was funded by the Center for Sustainable Materials Chemistry, a $20 million National Science Foundation-funded center co-based at the UO and Oregon State University in Corvallis (CHE-1102637). Co-authors with Trotochaud and Boettcher were James K. Ranney, an undergraduate student in chemistry, and Kerisha N. Williams, who participated under the NSF-funded Undergraduate Catalytic Outreach and Research Experiences (UCORE) program.
Funding for the research detailed in the second paper also came, in part, from the Center for Sustainable Materials Chemistry. The DOE grant to Boettcher also supported co-author Thomas J. Mills, a UO graduate.
####
About University of Oregon
The University of Oregon is among the 108 institutions chosen from 4,633 U.S. universities for top-tier designation of "Very High Research Activity" in the 2010 Carnegie Classification of Institutions of Higher Education. The UO also is one of two Pacific Northwest members of the Association of American Universities.
For more information, please click here
Contacts:
Jim Barlow
541-346-3481
Sources:
Shannon Boettcher
assistant professor of chemistry
541-346-2543
Lena Trotochaud
Copyright © University of Oregon
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() Solar Materials and Electrochemistry Laboratory:
Solar Materials and Electrochemistry Laboratory:
![]() Follow UO Science on Facebook:
Follow UO Science on Facebook:
![]() More UO Science/Research News:
More UO Science/Research News:
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Thin films
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Solar/Photovoltaic
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() Charged “molecular beasts” the basis for new compounds: Researchers at Leipzig University use “aggressive” fragments of molecular ions for chemical synthesis November 3rd, 2023
Charged “molecular beasts” the basis for new compounds: Researchers at Leipzig University use “aggressive” fragments of molecular ions for chemical synthesis November 3rd, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








