Home > Press > 2 problems in chemical catalysis solved: University of Jyvaskyla Department of Chemistry and NanoScience Center
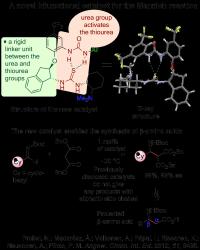 |
| This image shows a novel bifunctional catalyst for the Mannich reaction.
Credit: Professor Petri Pihko |
Abstract:
The research group of Professor Petri Pihko at the Department of Chemistry and the NanoScience Center of the University of Jyväskylä has solved two acute problems in chemical catalysis. The research has been funded by the Academy of Finland.
2 problems in chemical catalysis solved: University of Jyvaskyla Department of Chemistry and NanoScience Center
Finland | Posted on December 20th, 2012In the first project, the researchers designed a novel intramolecularly assisted catalyst for the synthesis of beta amino acids. Previously published catalysts work only with aromatic side chains in the imines, but the new catalyst designed at Jyväskylä does not have this limitation. The new method might find uses in the synthesis of beta amino acids, which are important building blocks for chemical biology. For the understanding of the catalytic mechanism and design of the catalyst, the researchers collaborated with the group of Imre Pápai (Hungarian Academy of Sciences, computational studies) and Academy Professor Kari Rissanen (Jyväskylä, X-ray characterisation of catalysts).
In the second project, the researchers identified a completely new mechanism for the amine-catalysed Michael addition reaction between aldehydes and nitroalkenes. The mechanism has been a source of intense discussion within the scientific community, with the groups of Professor Yujiro Hayashi (Tokyo), Professor Donna Blackmond (La Jolla, USA) and Professor Dieter Seebach (ETH, Switzerland) each presenting different possible mechanisms.
The new model proposed by the Pihko and Papai groups includes a new species, a six-membered ring, as the key on-cycle intermediate that is protonated in the rate-determining step. The work is a combination of computational and experimental studies that complement each other in understanding the mechanism and demonstrate how difficult mechanistic puzzles can be solved by joining the forces of both approaches.
The research results have been published in Angewandte Chemie.
####
For more information, please click here
Contacts:
Petri Pihko
358-505-289-132
Copyright © Academy of Finland
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








