Home > Press > New cobalt-graphene catalyst could challenge platinum for use in fuel cells: Performs nearly as well as precious metal catalysts
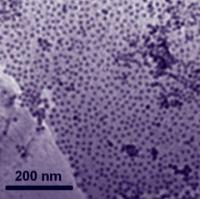 |
| Nanoparticles of cobalt attach themselves to a graphene substrate in a single layer. As a catalyst, the cobalt-graphene combination was a little slower getting the oxygen reduction reaction going, but it reduced oxygen faster and lasted longer than platinum-based catalysts.
Credit: Sun Lab/Brown University |
Abstract:
There's a new contender in the race to find an inexpensive alternative to platinum catalysts for use in hydrogen fuel cells.
New cobalt-graphene catalyst could challenge platinum for use in fuel cells: Performs nearly as well as precious metal catalysts
Providence, RI | Posted on October 17th, 2012Brown University chemist Shouheng Sun and his students have developed a new material — a graphene sheet covered by cobalt and cobalt-oxide nanoparticles — that can catalyze the oxygen reduction reaction nearly as well as platinum does and is substantially more durable.
The new material "has the best reduction performance of any nonplatinum catalyst," said Shaojun Guo, postdoctoral researcher in Sun's lab and lead author of a paper published online in the journal Angewandte Chemie International Edition.
The oxygen reduction reaction occurs on the cathode side of a hydrogen fuel cell. Oxygen functions as an electron sink, stripping electrons from hydrogen fuel at the anode and creating the electrical pull that keeps the current running through electrical devices powered by the cell. "The reaction requires a catalyst, and platinum is currently the best one," said Sun. "But it's very expensive and has a very limited supply, and that's why you don't see a lot of fuel cell use aside from a few special purposes."
Thus far scientists have been unable to develop a viable alternative. A few researchers, including Sun and Guo, have developed new catalysts that reduce the amount of platinum required, but an effective catalyst that uses no platinum at all remains elusive.
This new graphene-cobalt material is the most promising candidate yet, the researchers say. It is the first catalyst not made from a precious metal that comes close to matching platinum's properties.
Lab tests performed by Sun and his team showed that the new graphene-cobalt material was a bit slower than platinum in getting the oxygen reduction reaction started, but once the reaction was going, the new material actually reduced oxygen at a faster pace than platinum. The new catalyst also proved to be more stable, degrading much more slowly than platinum over time. After about 17 hours of testing, the graphene-cobalt catalyst was performing at around 70 percent of its initial capacity. The platinum catalyst the team tested performed at less than 60 percent after the same amount of time.
Cobalt is an abundant metal, readily available at a fraction of what platinum costs. Graphene is a one-atom-thick sheet of carbon atoms arranged in a honeycomb structure. Developed in the last few years, graphene is renowned for its strength, electrical properties, and catalytic potential.
Self-assembly process
Often, graphene nanoparticle materials are made by growing nanoparticles directly on the graphene surface. But that process is problematic for making a catalyst, Sun said. "It's really difficult to control the size, shape, and composition of nanoparticles," he said.
Sun and his team used a self-assembly method that gave them more control over the material's properties. First, they dispersed cobalt nanoparticles and graphene in separate solutions. The two solutions were then combined and pounded with sound waves to make sure they mixed thoroughly. That caused the nanoparticles to attach evenly to the graphene in a single layer, which maximizes the potential of each particle to be involved in the reaction. The material was then pulled out of solution using a centrifuge and dried. When exposed to air, outside layers of atomic cobalt on each nanoparticle are oxidized, forming a shell of cobalt-oxide that helps protect the cobalt core.
The researchers could control the thickness of the cobalt-oxide shell by heating the material at 70 degrees Celsius for varying amounts of time. Heating it longer increased the thickness of the shell. This way, they could fine-tune the structure in search of a combination that gives top performance. In this case, they found that a 1-nanometer shell of cobalt-oxide optimized catalytic properties.
Sun and his team are optimistic that with more study their material could one day be a suitable replacement for platinum catalysts. "Right now, it's comparable to platinum in an alkaline medium," Sun said, "but it's not ready for use yet. We still need to do more tests."
Ultimately, Sun says, finding a suitable nonplatinum catalyst is the key to getting fuel cells out of the laboratory phase and into production as power sources for cars and other devices.
####
For more information, please click here
Contacts:
Kevin Stacey
401-863-3766
Copyright © Brown University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Graphene/ Graphite
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
Self Assembly
![]() Liquid crystal templated chiral nanomaterials October 14th, 2022
Liquid crystal templated chiral nanomaterials October 14th, 2022
![]() Nanoclusters self-organize into centimeter-scale hierarchical assemblies April 22nd, 2022
Nanoclusters self-organize into centimeter-scale hierarchical assemblies April 22nd, 2022
![]() Atom by atom: building precise smaller nanoparticles with templates March 4th, 2022
Atom by atom: building precise smaller nanoparticles with templates March 4th, 2022
![]() Nanostructures get complex with electron equivalents: Nanoparticles of two different sizes break away from symmetrical designs January 14th, 2022
Nanostructures get complex with electron equivalents: Nanoparticles of two different sizes break away from symmetrical designs January 14th, 2022
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Automotive/Transportation
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








