Home > Press > Reversible doping: Hydrogen flips switch on vanadium oxide: Rice University physicists find reversible way to alter VO2's unique electronic about-face
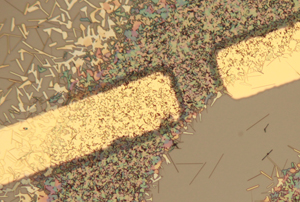 |
| Gold electrodes rest on clumps of vanadium oxide (VO2) wires that are each about 1,000 times smaller than a human hair. When baked in the presence of hydrogen gas, the wires next to the electrodes (dark region) absorb hydrogen and exhibit altered electronic behavior. CREDIT: Jiang Wei/Rice University |
Abstract:
If you are not a condensed matter physicist, vanadium oxide (VO2) may be the coolest material you've never heard of. It's a metal. It's an insulator. It's a window coating and an optical switch. And thanks to a new study by physicists at Rice University, scientists have a new way to reversibly alter VO2's electronic properties by treating it with one of the simplest substances -- hydrogen.
Reversible doping: Hydrogen flips switch on vanadium oxide: Rice University physicists find reversible way to alter VO2's unique electronic about-face
Houston, TX | Posted on May 21st, 2012So what is VO2? It's an oxidized form of the metal vanadium, an ingredient in hardened steel. When oxygen reacts with vanadium to form VO2, the atoms form crystals that look like long rectangular boxes. The vanadium atoms line up along the four edges of the box in regularly spaced rows. A single crystal of VO2 can have many of these boxes lined up side by side, and the crystals conduct electricity like wire as long as they are kept warm.
"The weird thing about this material is that if you cool it, when you get to 67 degrees Celsius, it goes through a phase transition that is both electronic and structural," said Rice's Douglas Natelson, lead co-author of the study in this week's Nature Nanotechnology. "Structurally, the vanadium atoms pair up and each pair is slightly canted, so you no longer have these long chains. When the phase changes, and these pairings take place, the material changes from being a electrical conductor to an electrical insulator."
While other materials exhibit a similar electronic about-face, VO2 is unique in that the change occurs at a relatively modest temperature -- around 153 degrees Fahrenheit -- and sometimes at incredible speed -- less than a trillionth of second. In recent years, scientists have put these quirky properties to work. In 2004, a group in London used VO2 to design a temperature-sensitive window coating that could absorb sunlight on cold days and turn reflective on hot days. And electronics researchers are also working to create optical switches from VO2.
"As an experimental physicist, VO2 is intriguing because the detailed physics of the material are still not well understood, and theoretical models alone cannot give us the answers," said Natelson, professor of physics and astronomy and of electrical and computer engineering at Rice. "Experiments are key to understanding this."
In 2010, Natelson and postdoctoral research associate Jiang Wei began to systematically study the phase changes in VO2. Wei and graduate student Heng Ji began by using a process called vapor deposition to grow VO2 wires that were about 1,000 times smaller than a human hair. One set of experiments on wires that had been baked in the presence of hydrogen gas returned particularly odd readings. Wei, Ji and Natelson determined that the hydrogen was apparently modifying the VO2 nanowires, but only those in contact with metal electrodes.
"The gold electrodes we were using to supply current to the experiment were acting as a catalyst that split the hydrogen gas molecules into atomic hydrogen, which could then diffuse into channels in the VO2," Natelson said. "It appears that the hydrogen is taken up into the VO2 crystals, and this changes their electronic properties. If a little hydrogen is added, the phase transition happens at a slightly lower temperature, and the insulating phase becomes more conductive. If enough hydrogen is added, the transition to the insulating phase disappears altogether."
To gain insight into just how the hydrogen is able to alter the transition, the experimenters consulted with theoretical physicist Andriy Nevidomskyy, assistant professor of physics and astronomy at Rice. Nevidomskyy's calculations showed that the hydrogen changes the amount of charge in the VO2 material and also forces the crystal to expand slightly. Both of these effects favor the metallic state.
This is not the first time physicists have lowered the transition temperature of VO2 by adding other materials -- a technique known as "doping." But Natelson said Rice's hydrogen doping is unique in that it is completely reversible: To remove the hydrogen, the material simply has to be baked in an oven at moderate temperature.
"On the applied side, there may be a number of applications for this, like ultrasensitive hydrogen sensors," Natelson said. "But the more immediate payoff will likely be in helping us to better understand the physics involved in the VO2 phase transition. If we can find out exactly how much hydrogen is required to shut down the transition, then we will have a knob that we can turn to systematically raise or lower the temperature in future experiments."
Research co-authors include Natelson, Wei, Ji, Nevidomskyy and Wenhua Guo, transmission electron microscope manager at Rice's Shared Equipment Authority. The research was funded by the Department of Energy's Office of Science and an Evans Atwell/Welch postdoctoral fellowship from the Robert A. Welch Foundation.
####
About Rice University
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation's top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is known for its "unconventional wisdom." With 3,708 undergraduates and 2,374 graduate students, Rice's undergraduate student-to-faculty ratio is 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice has been ranked No. 1 for best quality of life multiple times by the Princeton Review and No. 4 for "best value" among private universities by Kiplinger's Personal Finance. To read "What they're saying about Rice," go to www.rice.edu/nationalmedia/Rice.pdf.
For more information, please click here
Contacts:
David Ruth
713-348-6327
Jade Boyd
713-348-6778
Copyright © Rice University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() A copy of the Nature Nanomaterials paper is available at:
A copy of the Nature Nanomaterials paper is available at:
| Related News Press |
Physics
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chip Technology
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
![]() HKUST researchers develop new integration technique for efficient coupling of III-V and silicon February 16th, 2024
HKUST researchers develop new integration technique for efficient coupling of III-V and silicon February 16th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Materials/Metamaterials/Magnetoresistance
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








