Home > Press > Nanosheet Catalyst Discovered to Sustainably Split Hydrogen from Water: A new low-cost non-noble electrocatalyst efficiently generates hydrogen gas for fuel
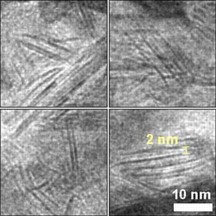 |
| This magnified image from a transmission electron microscope reveals details of the unexpected nanosheet structure of the nickel-molybdenum-nitride catalyst, seen here as dark, straight lines. |
Abstract:
Hydrogen gas offers one of the most promising sustainable energy alternatives to limited fossil fuels. But traditional methods of producing pure hydrogen face significant challenges in unlocking its full potential, either by releasing harmful carbon dioxide into the atmosphere or requiring rare and expensive chemical elements such as platinum.
Nanosheet Catalyst Discovered to Sustainably Split Hydrogen from Water: A new low-cost non-noble electrocatalyst efficiently generates hydrogen gas for fuel
Upton, NY | Posted on May 9th, 2012Now, scientists at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory have developed a new electrocatalyst that addresses one of these problems by generating hydrogen gas from water cleanly and with much more affordable materials. The novel form of catalytic nickel-molybdenum-nitride - described in a paper published online May 8, 2012 in the journal Angewandte Chemie International Edition - surprised scientists with its high-performing nanosheet structure, introducing a new model for effective hydrogen catalysis.
"We wanted to design an optimal catalyst with high activity and low costs that could generate hydrogen as a high-density, clean energy source," said Brookhaven Lab chemist Kotaro Sasaki, who first conceived the idea for this research. "We discovered this exciting compound that actually outperformed our expectations."
Goldilocks chemistry
Water provides an ideal source of pure hydrogen - abundant and free of harmful greenhouse gas byproducts. The electrolysis of water, or splitting water (H2O) into oxygen (O2) and hydrogen (H2), requires external electricity and an efficient catalyst to break chemical bonds while shifting around protons and electrons. To justify the effort, the amount of energy put into the reaction must be as small as possible while still exceeding the minimum required by thermodynamics, a figure associated with what is called overpotential.
For a catalyst to facilitate an efficient reaction, it must combine high durability, high catalytic activity, and high surface area. The strength of an element's bond to hydrogen determines its reaction level - too weak, and there's no activity; too strong, and the initial activity poisons the catalyst.
"We needed to create high, stable activity by combining one non-noble element that binds hydrogen too weakly with another that binds too strongly," said James Muckerman, the senior chemist who led the project. "The result becomes this well-balanced Goldilocks compound - just right."
Unfortunately, the strongest traditional candidate for an electrocatlytic Goldilocks comes with a prohibitive price tag.
Problems with platinum
Platinum is the gold standard for electrocatalysis, combining low overpotential with high activity for the chemical reactions in water-splitting. But with rapidly rising costs - already hovering around $50,000 per kilogram - platinum and other noble metals discourage widespread investment.
"People love platinum, but the limited global supply not only drives up price, but casts doubts on its long-term viability," Muckerman said. "There may not be enough of it to support a global hydrogen economy."
In contrast, the principal metals in the new compound developed by the Brookhaven team are both abundant and cheap: $20 per kilogram for nickel and $32 per kilogram for molybdenum. Combined, that's 1000 times less expensive than platinum. But with energy sources, performance is often a more important consideration than price.
Turning nickel into platinum
In this new catalyst, nickel takes the reactive place of platinum, but it lacks a comparable electron density. The scientists needed to identify complementary elements to make nickel a viable substitute, and they introduced metallic molybdenum to enhance its reactivity. While effective, it still couldn't match the performance levels of platinum.
"We needed to introduce another element to alter the electronic states of the nickel-molybdenum, and we knew that nitrogen had been used for bulk materials, or objects larger than one micrometer," said research associate Wei-Fu Chen, the paper's lead author. "But this was difficult for nanoscale materials, with dimensions measuring billionths of a meter."
The scientists expected the applied nitrogen to modify the structure of the nickel-molybdenum, producing discrete, sphere-like nanoparticles. But they discovered something else.
Subjecting the compound to a high-temperature ammonia environment infused the nickel-molybdenum with nitrogen, but it also transformed the particles into unexpected two-dimensional nanosheets. The nanosheet structures offer highly accessible reactive sites - consider the surface area difference between bed sheets laid out flat and those crumpled up into balls - and therefore more reaction potential.
Using a high-resolution transmission microscope in Brookhaven Lab's Condensed Matter Physics and Materials Science Department, as well as x-ray probes at the National Synchrotron Light Source, the scientists determined the material's 2D structure and probed its local electronic configurations.
"Despite the fact that metal nitrides have been extensively used, this is the first example of one forming a nanosheet," Chen said. "Nitrogen made a huge difference - it expanded the lattice of nickel-molybdenum, increased its electron density, made an electronic structure approaching that of noble metals, and prevented corrosion."
Hydrogen future
The new catalyst performs nearly as well as platinum, achieving electrocatalytic activity and stability unmatched by any other non-noble metal compounds. "The production process is both simple and scalable," Muckerman said, "making nickel-molybdenum-nitride appropriate for wide industrial applications."
While this catalyst does not represent a complete solution to the challenge of creating affordable hydrogen gas, it does offer a major reduction in the cost of essential equipment. The team emphasized that the breakthrough emerged through fundamental exploration, which allowed for the surprising discovery of the nanosheet structure.
"Brookhaven Lab has a very active fuel cell and electrocatalysis group," Muckerman said. "We needed to figure out fundamental approaches that could potentially be game-changing, and that's the spirit in which we're doing this work. It's about coming up with a new paradigm that will guide future research."
Additional collaborators on this research were: Anatoly Frenkel of Yeshiva University, Nebojsa Marinkovic of the University of Delaware, and Chao Ma, Yimei Zhu and Radoslav Adzic of Brookhaven Lab.
The research was funded by Brookhaven's Laboratory Directed Research and Development (LDRD) Program. The National Sychrotron Light Source and other Brookhaven user facilities are supported by the DOE Office of Science.
DOE's Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
####
About Brookhaven National Laboratory
One of ten national laboratories overseen and primarily funded by the Office of Science of the U.S. Department of Energy (DOE), Brookhaven National Laboratory conducts research in the physical, biomedical, and environmental sciences, as well as in energy technologies and national security. Brookhaven Lab also builds and operates major scientific facilities available to university, industry and government researchers. Brookhaven is operated and managed for DOE's Office of Science by Brookhaven Science Associates, a limited-liability company founded by the Research Foundation of State University of New York on behalf of Stony Brook University, the largest academic user of Laboratory facilities, and Battelle, a nonprofit, applied science and technology organization. Visit Brookhaven Lab's electronic newsroom for links, news archives, graphics, and more at www.bnl.gov/newsroom , or follow Brookhaven Lab on Twitter, twitter.com/BrookhavenLab .
For more information, please click here
Contacts:
Justin Eure
(631) 344-2347
or
Peter Genzer
(631) 344-3174
Copyright © Brookhaven National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
![]() Three-pronged approach discerns qualities of quantum spin liquids November 17th, 2023
Three-pronged approach discerns qualities of quantum spin liquids November 17th, 2023
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Environment
![]() Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Research partnerships
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() 'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








