Home > Press > Touch of gold improves nanoparticle fuel-cell reactions
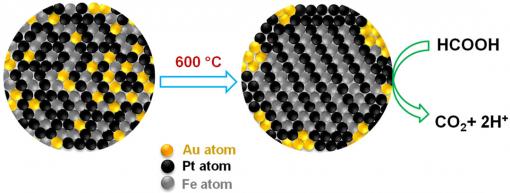 |
| Midas touch on the nanoscale Gold atoms create orderly places for iron and platinum atoms, then retreat to the periphery of the fuel cell, where they scrub carbon monoxide from fuel reactions. The tighter organization and cleaner reactions extend the cell's performance life. Credit: Sun Lab/Brown University |
Abstract:
Chemists at Brown University have created a triple-headed metallic nanoparticle that reportedly performs better and lasts longer than any other nanoparticle catalyst studied in fuel-cell reactions. The key is the addition of gold: It yields a more uniform crystal structure while removing carbon monoxide from the reaction. Results published in the Journal of the American Chemical Society.
Touch of gold improves nanoparticle fuel-cell reactions
Providence, RI | Posted on March 12th, 2012Advances in fuel-cell technology have been stymied by the inadequacy of metals studied as catalysts. The drawback to platinum, other than cost, is that it absorbs carbon monoxide in reactions involving fuel cells powered by organic materials like formic acid. A more recently tested metal, palladium, breaks down over time.
Now chemists at Brown University have created a triple-headed metallic nanoparticle that they say outperforms and outlasts all others at the anode end in formic-acid fuel-cell reactions. In a paper published in the Journal of the American Chemical Society, the researchers report a 4-nanometer iron-platinum-gold nanoparticle (FePtAu), with a tetragonal crystal structure, generates higher current per unit of mass than any other nanoparticle catalyst tested. Moreover, the trimetallic nanoparticle at Brown performs nearly as well after 13 hours as it did at the start. By contrast, another nanoparticle assembly tested under identical conditions lost nearly 90 percent of its performance in just one-quarter of the time.
"We've developed a formic acid fuel-cell catalyst that is the best to have been created and tested so far," said Shouheng Sun, chemistry professor at Brown and corresponding author on the paper. "It has good durability as well as good activity."
Gold plays key roles in the reaction. First, it acts as a community organizer of sorts, leading the iron and platinum atoms into neat, uniform layers within the nanoparticle. The gold atoms then exit the stage, binding to the outer surface of the nanoparticle assembly. Gold is effective at ordering the iron and platinum atoms because the gold atoms create extra space within the nanoparticle sphere at the outset. When the gold atoms diffuse from the space upon heating, they create more room for the iron and platinum atoms to assemble themselves. Gold creates the crystallization chemists want in the nanoparticle assembly at lower temperature.
Gold also removes carbon monoxide (CO) from the reaction by catalyzing its oxidation. Carbon monoxide, other than being dangerous to breathe, binds well to iron and platinum atoms, gumming up the reaction. By essentially scrubbing it from the reaction, gold improves the performance of the iron-platinum catalyst. The team decided to try gold after reading in the literature that gold nanoparticles were effective at oxidizing carbon monoxide — so effective, in fact, that gold nanoparticles had been incorporated into the helmets of Japanese firefighters. Indeed, the Brown team's triple-headed metallic nanoparticles worked just as well at removing CO in the oxidation of formic acid, although it is unclear specifically why.
The authors also highlight the importance of creating an ordered crystal structure for the nanoparticle catalyst. Gold helps researchers get a crystal structure called "face-centered-tetragonal," a four-sided shape in which iron and platinum atoms essentially are forced to occupy specific positions in the structure, creating more order. By imposing atomic order, the iron and platinum layers bind more tightly in the structure, thus making the assembly more stable and durable, essential to better-performing and longer-lasting catalysts.
In experiments, the FePtAu catalyst reached 2809.9 mA/mg Pt (mass-activity, or current generated per milligram of platinum), "which is the highest among all NP (nanoparticle) catalysts ever reported," the Brown researchers write. After 13 hours, the FePtAu nanoparticle has a mass activity of 2600mA/mg Pt, or 93 percent of its original performance value. In comparison, the scientists write, the well-received platinum-bismuth nanoparticle has a mass activity of about 1720mA/mg Pt under identical experiments, and is four times less active when measured for durability.
The researchers note that other metals may be substituted for gold in the nanoparticle catalyst to improve the catalyst's performance and durability.
"This communication presents a new structure-control strategy to tune and optimize nanoparticle catalysis for fuel oxidations," the researchers write.
Sen Zhang, a third-year graduate student in Sun's lab, helped with the nanoparticle design and synthesis. Shaojun Guo, a postdoctoral fellow in Sun's lab performed electrochemical oxidation experiments. Huiyuan Zhu, a second-year graduate student in Sun's lab, synthesized the FePt nanoparticles and ran control experiments. The other contributing author is Dong Su from the Center for Functional Nanomaterials at Brookhaven National Laboratory, who analyzed the structure of the nanoparticle catalyst using the advanced electron microscopy facilities there.
The U.S. Department of Energy and the Exxon Mobil Corporation funded the research.
####
For more information, please click here
Contacts:
Richard Lewis
401-863-3766
Copyright © Brown University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Imaging
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Tools
![]() Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








