Home > Press > 2 for 1: Simultaneous size and electrochemical measurement of nanomaterials
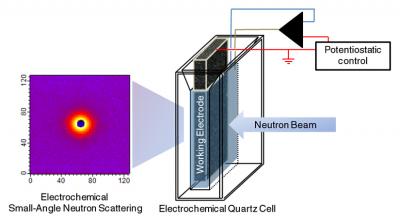 |
| Schematic of NIST's "eSANS" (electrochemical Small-Angle Neutron Scattering) cell. A highly porous, sponge-like carbon electrode maximizes surface area for electrochemical reactions while structural details like particle size and configuration are measured using neutron scattering (image at left).
Credit: Prabhu/NIST |
Abstract:
Researchers at the National Institute of Standards and Technology (NIST) have done a mash-up of two very different experimental techniques—neutron scattering and electrochemical measurements—to enable them to observe structural changes in nanoparticles as they undergo an important type of chemical reaction. Their recently published technique* allows them to directly match up particle size, shape and agglomeration with the "redox" chemical properties of the particles. The measurements are important both for the design of nanoparticles for particular applications and for toxicology studies.
2 for 1: Simultaneous size and electrochemical measurement of nanomaterials
Gaithersburg, MD | Posted on March 7th, 2012Nanoparticles present unique engineering challenges—and opportunities—because their extremely small size can give them physical properties quite unlike those they have in bulk quantities. The challenge for materials scientists is to determine just what those changes are and how they relate to particle size and structure.
The NIST team was interested in the oxidation-reduction—redox— properties of zinc oxide nanoparticles, which are used or being considered for a wide variety of applications ranging from sunscreens and antibacterial coatings to semiconductor and photoelectronic devices.
Redox reactions are one of the major divisions of chemical reactions, those that involve a transfer of electrons from one atom or molecule to another. Redox properties determine the path a chemical reaction will take. "They are the drivers of many biological processes," explains NIST materials researcher Vivek Prabhu. "There are many biochemical reactions that are well-defined oxidation-reduction reactions. There are tables of these. But there are no such tables that we know of on how nanoparticles can affect those reactions."
The NIST team knew they could monitor the size, shape and dispersion of nanoparticles in solution using SANS—small-angle neutron scattering. The scattering patterns from a SANS instrument, says Prabhu, give you not only those details but structural information about the solution itself, the size distribution of the particles and whether they clump together, all in "real" time as the experiment progresses.
Redox properties, on the other hand, are measured in electrochemical cells that are essentially half of a battery. Voltage and the amount of current flowing through the primary electrode depend on the reaction redox potential and the concentration of the test material.
The problem, Prabhu explains, is that SANS measures things in bulk, in a volume of space, but, "An electrochemical experiment is a very local experiment—it happens at an interface. What we needed was to maximize the interface." The answer, contributed by his partner, Vytas Reipa, is an exotic material called reticulated glassy carbon. "Like a very stiff household sponge or scouring pad made of pure carbon," Prabhu explains. The porous carbon electrode turned out to be an ideal terminal—lots of surface area to serve as a reaction interface; nearly transparent to neutrons, so it doesn't contribute much background noise; and best of all, it works well in water, enabling the study of nanoparticles in aqueous solutions, critical for biological reactions.
A big advantage of the "eSANS" technique, Prabhu says, is its generality. "You can apply our method to nearly any dispersed material that is of interest to redox chemistry—polymers, redox proteins, nucleic acids—at this nanoscale. Small polymer chains, for example. You can't really see them with electron microscopy, you can with neutrons."
* V.M. Prabhu and V. Reipa. In situ electrochemical small-angle neutron scattering (eSANS) for quantitative structure and redox properties of nanoparticles. J. Phys. Chem. Lett. 2012, 3, 646-650 dx.doi.org/10.1021/jz300124t.
####
For more information, please click here
Contacts:
Michael Baum
301-975-2763
Copyright © National Institute of Standards and Technology (NIST)
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
![]() Three-pronged approach discerns qualities of quantum spin liquids November 17th, 2023
Three-pronged approach discerns qualities of quantum spin liquids November 17th, 2023
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Tools
![]() Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








