Home > Press > Study: Honeycomb structure responsible for bacteria's extraordinary sense
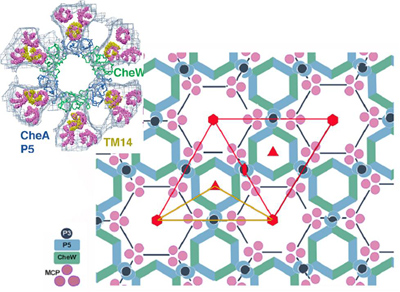 |
| Courtesy of Crane Lab Honeycomb-like hexagonal lattice formed by the network of receptor molecules (pink), associated enzymes (blue) and coupling proteins (green) in motile bacteria. Such a cooperative lattice arranged across their cell membranes helps bacteria sense their environment with extreme sensitivity. Inset shows the structure of a unit cell of the lattice in detail (top left). |
Abstract:
Cornell researchers have peered into the complex molecular network of receptors that give one-celled organisms like bacteria the ability to sense their environment and respond to chemical changes as small as 1 part in 1,000.
Study: Honeycomb structure responsible for bacteria's extraordinary sense
Ithaca, NY | Posted on February 20th, 2012Just as humans use five senses to navigate through surroundings, bacteria employ an intricate structure of thousands of receptor molecules, associated enzymes and linking proteins straddling their cell membranes that trigger responses to external chemical changes.
Researchers in the lab of Brian Crane, professor of chemistry and chemical biology, with collaborators in the lab of Grant Jensen at the California Institute of Technology, have mapped out the honeycomb-like hexagonal arrangement of these receptor complexes in unprecedented detail.
They report their discovery in the Feb. 20 issue of the Proceedings of the National Academy of Sciences.
"The highlight of the paper is that by using a combination of [techniques], we've been able to image these complex arrays in live cells and determine how they are structured -- they are a very unique biological assembly that is conserved across nearly all classes of motile bacteria," said Crane.
The findings might have potential applications in engineering biologically inspired synthetic molecular devices to detect specific chemicals with high sensitivity over a wide dynamic range; they also could shed light on the pathogenesis of various bacterial diseases like syphilis, cholera and Lyme disease. They may also give some insight into the functioning of the human immune system, where special cells may be employing similar cooperative networks of receptors to recognize and fight against foreign antigens, Crane said.
Scientists are trying to determine how one receptor -- on detecting nutrients, oxygen or acidity, for example -- triggers communication through multiple enzymes in the complex, setting off a chain reaction. This cooperation leads to a gain or amplification in the signal that is finally communicated to the tails (flagella) of the bacterium, affecting the way they spin. Such a response allows the bacterium to move toward food sources or away from toxic environments.
The latest work takes a big step toward understanding this mechanism by demonstrating for the first time how the individual pieces of receptors, enzymes and coupling proteins fit together to generate an extended network. It builds on previous work in the Crane lab done in collaboration with Jack Freed, Cornell professor of chemistry and chemical biology.
"We were able to see how the enzymes and proteins are linked together in the complex array and also their interactions with the receptors," said Xiaoxiao Li, graduate student and joint lead author on the study.
High-resolution X-ray images of the bacterial membrane receptor complex were obtained after extraction, purification and crystallization at the Cornell High Energy Synchrotron Source (CHESS). The scientists reconstructed the complicated molecular structure of the complex with an accuracy that is within a nanometer (a billionth of a meter). Pictures of the structure were also captured inside living cells, by first "flash-freezing" the cells and then scattering electrons off them.
The researchers deduced that trimers (groups of three) of receptor molecules are arranged at the vertices of hexagons surrounding rings of enzymes and coupling proteins. These rings linked by proteins form the backbone of the extended honeycomb-like network.
The research was funded by the Howard Hughes Medical Institute, the National Institutes of Health, and the Gordon and Betty Moore Foundation.
Graduate student Vivek Venkataraman is a writer intern for the Cornell Chronicle.
####
For more information, please click here
Contacts:
Media Contact:
John Carberry
(607) 255-5353
Cornell Chronicle:
Krishna Ramanujan
(607) 255-3290
Copyright © Cornell University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Nanomedicine
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
![]() Researchers develop artificial building blocks of life March 8th, 2024
Researchers develop artificial building blocks of life March 8th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Nanobiotechnology
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
![]() Researchers develop artificial building blocks of life March 8th, 2024
Researchers develop artificial building blocks of life March 8th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








