Home > Press > New medical, research tool possible by probing cell mechanics
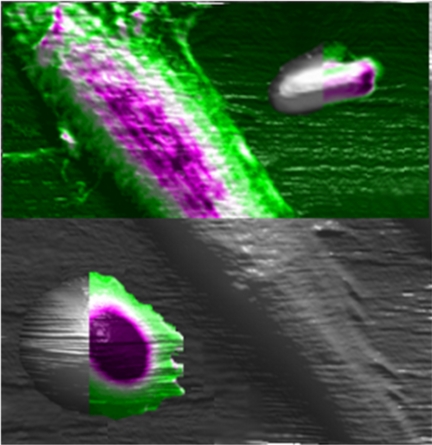 |
| This artist's conception depicts the use of an atomic force microscope to study the mechanical properties of cells, an innovation that might result in a new way to diagnose disease and study biological processes. Here, three types of cells are studied using the instrument: a rat fibroblast is the long slender cell in the center, an E coli bacterium is at the top right and a human red blood cell is at the lower left. The colored portions show the benefit of the new technique, representing the mechanical properties of the cells, whereas the gray portions represent what was possible using a conventional approach. (Purdue University image/Alexander Cartagena) |
Abstract:
Mapping Nanomechanical Properties of Live Cells
Using Multi-harmonic Atomic Force Microscopy
A. Raman1,3 †*, S. Trigueros2 †, A. Cartagena1,3, A. P. Z. Stevenson2,
M. Susilo1, E. Nauman1,4 and S. Antoranz Contera2
1School of Mechanical Engineering, Purdue University
2Department of Physics and Institute of Nanoscience for Medicine,
Oxford Martin School, University of Oxford
3Birck Nanotechnology Center, Purdue University,
4Weldon School of Biomedical Engineering
The nanomechanical properties of living cells, such as their surface elastic response and adhesion, have important roles in cellular processes such as morphogenesis1, mechano-transduction2, focal adhesion3, motility4,5, metastasis6 and drug delivery7-10. Techniques based on quasi-static atomic force microscopy techniques11-17 can map these properties, but they lack the spatial and temporal resolution that is needed to observe many of the relevant details. Here, we present a dynamic atomic force microscopy18-28 method to map quantitatively the nanomechanical properties of live cells with a throughput (measured in pixels/minute) that is 10-1,000 times higher than that achieved with quasi-static atomic force microscopy techniques. The local properties of a cell are derived from the 0th, 1st and 2nd harmonic components of the Fourier spectrum of the AFM cantilevers interacting with the cell surface. Local stiffness, stiffness gradient and the viscoelastic dissipation of live Escherichia coli bacteria, rat ?broblasts and human red blood cells were all mapped in buffer solutions. Our method is compatible with commercial atomic force microscopes and could be used to analyze mechanical changes in tumors, cells and bio?lm formation with sub-10 nm detail.
New medical, research tool possible by probing cell mechanics
West Lafayette, IN | Posted on November 21st, 2011Researchers are making progress in developing a system that measures the mechanical properties of living cells, a technology that could be used to diagnose human disease and better understand biological processes.
The team used an instrument called an atomic force microscope to study three distinctly different types of cells to demonstrate the method's potentially broad applications, said Arvind Raman, a Purdue University professor of mechanical engineering.
For example, the technique could be used to study how cells adhere to tissues, which is critical for many disease and biological processes; how cells move and change shape; how cancer cells evolve during metastasis; and how cells react to mechanical stimuli needed to stimulate production of vital proteins. The technique could be used to study the mechanical properties of cells under the influence of antibiotics and drugs that suppress cancer to learn more about the mechanisms involved.
Findings have been posted online in the journal Nature Nanotechnology and will appear in the December print issue. The work involves researchers from Purdue and the University of Oxford.
"There's been a growing realization of the role of mechanics in cell biology and indeed a lot of effort in building models to explain how cells feel, respond and communicate mechanically both in health and disease," said Sonia Contera, a paper co-author and director of the Oxford Martin Programme on Nanotechnology and an academic fellow at Oxford physics. "With this paper, we provide a tool to start addressing some of these questions quantitatively: This is a big step."
An atomic force microscope uses a tiny vibrating probe to yield information about materials and surfaces on the scale of nanometers, or billionths of a meter. Because the instrument enables scientists to "see" objects far smaller than possible using light microscopes, it could be ideal for "mapping" the mechanical properties of the tiniest cellular structures.
"The maps identify the mechanical properties of different parts of a cell, whether they are soft or rigid or squishy," said Raman, who is working with doctoral student Alexander Cartagena and other researchers. "The key point is that now we can do it at high resolution and higher speed than conventional techniques."
The high-speed capability makes it possible to watch living cells and observe biological processes in real time. Such a technique offers the hope of developing a "mechanobiology-based" assay to complement standard biochemical assays.
"The atomic force microscope is the only tool that allows you to map the mechanical properties - take a photograph, if you will - of the mechanical properties of a live cell," Raman said.
However, existing techniques for mapping these properties using the atomic force microscope are either too slow or don't have high enough resolution.
"This innovation overcomes those limitations, mostly through improvements in signal processing," Raman said. "You don't need new equipment, so it's an economical way to bump up pixels per minute and get quantitative information. Most importantly, we applied the technique to three very different kinds of cells: bacteria, human red blood cells and rat fibroblasts. This demonstrates its potential broad utility in medicine and research."
The technique is nearly five times faster than standard atomic force microscope techniques.
The Nature Nanotechnology paper was written by Raman; Cartagena; Sonia Trigueros, a Senior Research Fellow in the Oxford Martin Programme on Nanotechnology; Oxford doctoral student Amadeus Stevenson; Purdue instructor Monica Susilo; Eric Nauman, an associate professor of mechanical engineering; and Contera.
The National Science Foundation and Engineering and Physical Sciences Research Council of the U.K. funded the research.
####
For more information, please click here
Contacts:
Writer:
Emil Venere
765-494-4709
Sources:
Arvind Raman
765-494-5733
Alexander Cartagena
Copyright © Purdue University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Imaging
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
![]() Observation of left and right at nanoscale with optical force October 6th, 2023
Observation of left and right at nanoscale with optical force October 6th, 2023
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Nanomedicine
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
![]() Researchers develop artificial building blocks of life March 8th, 2024
Researchers develop artificial building blocks of life March 8th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Tools
![]() Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








