Home > Press > Fluoride shuttle increases storage capacity: KIT researchers develop new concept for rechargeable batteries
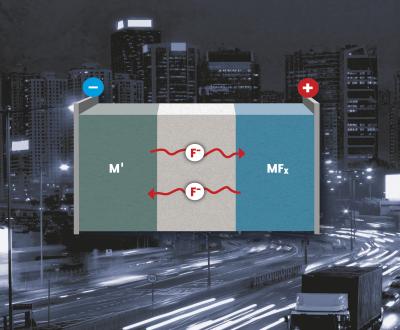 |
| A fluoride-containing electrolyte separates the metal anode from the metal fluoride cathode.
Credit: KIT |
Abstract:
KIT researchers have developed a new concept for rechargeable batteries. Based on a fluoride shuttle -- the transfer of fluoride anions between the electrodes -- it promises to enhance the storage capacity reached by lithium-ion batteries by several factors. Operational safety is also increased, as it can be done without lithium. The fluoride-ion battery is presented for the first time in the Journal of Materials Chemistry by Dr. Maximilian Fichtner and Dr. Munnangi Anji Reddy.
Fluoride shuttle increases storage capacity: KIT researchers develop new concept for rechargeable batteries
Berlin, Germany | Posted on October 21st, 2011Lithium-ion batteries are applied widely, but their storage capacity is limited. In the future, battery systems of enhanced energy density will be needed for mobile applications in particular. Such batteries can store more energy at reduced weight. For this reason, KIT researchers are also conducting research into alternative systems. A completely new concept for secondary batteries based on metal fluorides was developed by Dr. Maximilian Fichtner, Head of the Energy Storage Systems Group, and Dr. Munnangi Anji Reddy at the KIT Institute of Nanotechnology (INT).
Metal fluorides may be applied as conversion materials in lithium-ion batteries. They also allow for lithium-free batteries with a fluoride-containing electrolyte, a metal anode, and metal fluoride cathode, which reach a much better storage capacity and possess improved safety properties. Instead of the lithium cation, the fluoride anion takes over charge transfer. At the cathode and anode, a metal fluoride is formed or reduced. "As several electrons per metal atom can be transferred, this concept allows to reach extraordinarily high energy densities - up to ten times as high as those of conventional lithium-ion batteries," explains Dr. Maximilian Fichtner.
The KIT researchers are now working on the further development of material design and battery architecture in order to improve the initial capacity and cyclic stability of the fluoride-ion battery. Another challenge lies in the further development of the electrolyte: The solid electrolyte applied so far is suited for applications at elevated temperatures only. It is therefore aimed at finding a liquid electrolyte that is suited for use at room temperature.
M. Anji Reddy and M. Fichtner: Batteries based on fluoride shuttle. Journal of Materials Chemistry. 2011, Advance Article. DOI: 10.1039/C1JM13535J.
####
About Helmholtz Association of German Research Centres
Karlsruhe Institute of Technology (KIT) is a public corporation according to the legislation of the state of Baden-Württemberg. It fulfills the mission of a university and the mission of a national research center of the Helmholtz Association. KIT focuses on a knowledge triangle that links the tasks of research, teaching, and innovation.
For more information, please click here
Contacts:
Monika Landgraf
49-721-608-47414
Copyright © Helmholtz Association of German Research Centres
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








