Home > Press > Researchers Begin Testing of Promising New Nanomaterial for Hydrogen Storage
 |
| Rensselaer/Liu
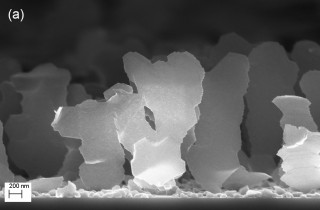
Nanoblades |
Abstract:
Scientists at Rensselaer Polytechnic Institute are working to optimize a promising new nanomaterial called nanoblades for use in hydrogen storage. During their testing of the new material, they have discovered that it can store and release hydrogen extremely fast and at low temperatures compared to similar materials. Another important aspect of the new material is that it is also rechargeable. These attributes could make it ideal for use in onboard hydrogen storage for next-generation hydrogen or fuel cell vehicles.
Researchers Begin Testing of Promising New Nanomaterial for Hydrogen Storage
Troy, NY | Posted on September 14th, 2011The findings on the performance of the nanoblades are published in the September 2011 edition of The International Journal of Hydrogen Energy in an article titled "Low-temperature cycling of hydrogenation-dehydrogenation of Pd-decorated Mg nanoblades." The research is sponsored by the National Science Foundation.
The scientists created the magnesium-based nanoblades for the first time in 2007. Unlike three-dimensional nanosprings and rods, nanoblades are asymmetric. They are extremely thin in one dimension and wide in another dimension, creating very large surface areas. They also are spread out with up to one micron in between each blade.
In order to store hydrogen, a large surface area with space in between nanostructures is needed to provide room for the material to expand as more hydrogen atoms are stored. The vast surface area and ultrathin profile of each nanoblade, coupled with the spaces between each blade, could make them ideal for this application, according to Gwo-Ching Wang, professor of physics, applied physics, and astronomy at Rensselaer.
To create the nanoblades, the researchers use oblique angle vapor deposition. This fabrication technique builds nanostructures by vaporizing a material — magnesium in this case — and allowing the vaporized atoms to deposit on a surface at an oblique angle. The finished material is then decorated with a metallic catalyst to trap and store hydrogen. For this research, the nanoblades were coated with palladium.
In their most recent paper, the researchers report on their tests of the nanoblades' performance. Understanding how the material responds to hydrogen over time is essential to improving the material for future use in hydrogen vehicles, according to postdoctoral researcher and lead author of the new paper Yu Liu.
"The requirements from the Department of Energy are very challenging for existing hydrogen storage technology, particularly when it comes to new energy storage materials for onboard hydrogen storage," said Liu. "All new materials must operate at low temperatures, desorb hydrogen quickly, be cost efficient, and be recyclable."
Their work with nanoblades is already showing promise in all these areas, according to Wang and Liu.
What they found is that the nanoblades began releasing hydrogen at 340 degrees K (approximately 67 degrees Celsius). When the temperature was increased slightly to 373 K (100 degrees C), the hydrogen stored in the nanoblades was released in just 20 minutes. Many other materials require more than double that temperature to operate at that rate, according to Liu.
They also found that the nanoblades are recyclable. This means that they can be recharged after hydrogen release and used over and over. Such reusability is essential for practical applications.
Using a technique called reflection high-energy electron diffraction (RHEED) and temperature programmed desorption (TPD) — which are equipped onto an integrated ultrahigh vacuum system with a combination of a high-pressure reaction cell and a thin-film deposition chamber — they found that the current nanoblades can go through more than 10 cycles of hydrogen absorption and release.
The RHEED technique is different from other processes, such as X-ray diffraction, because it monitors the near surface structure, phase, and grain size of the material as it changes. Tracking the surface evolution of the material provides insight into how the structure evolves over time.
Using RHEED, they found that over time the catalyst becomes poisoned and the magnesium reacts with oxygen. This causes oxidation, which ultimately degrades the material causing both morphological and chemical changes to the material.
They will now work to optimize the material with different catalysts and polymer protective coatings to improve performance and increase the number of cycles that the material can go through without degradation.
"The next steps are to improve recyclability," Wang said. "We have found the root cause of the degradation of the material; now we can begin to improve the material."
Wang and Liu were joined in the research by Professor of Physics, Applied Physics, and Astronomy Toh-Ming Lu and doctoral student Liang Chen. This experimental work received theoretical insights provided by the Gail and Jeffrey L. Kodosky '70 Senior Constellation Professor of Physics, Information Technology, and Entrepreneurship Shengbai Zhang and doctoral student Wieyu Xie.
####
For more information, please click here
Contacts:
Gabrielle DeMarco
Rensselaer Polytechnic Institute
(518) 276-6542
Copyright © Newswise
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Materials/Metamaterials/Magnetoresistance
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








