Home > Press > Chemists Create Molecular "Flasks": Researchers design a self-assembling material that can house other molecules
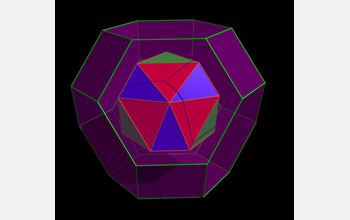 |
| This image is a simplified representation of a compound (red, blue and green) nesting inside a single truncated octahedron (purple).
Credit: Michael D. Ward, New York University |
Abstract:
Chemical reactions happen all of the time: Some things burn or rust; others react to light exposure. Even batteries use chemical reactions to supply electricity. One of the big challenges chemists continually face is finding new ways to control these reactions or create conditions that promote desirable reactions and limit undesirable ones.
Chemists Create Molecular "Flasks": Researchers design a self-assembling material that can house other molecules
Arlington, VA | Posted on July 21st, 2011Recently, researchers at New York University (NYU) demonstrated an ability to make new materials with empty space on the inside, an advancement that could potentially control desired and unwanted chemical reactions.
Mike Ward, of NYU's department of chemistry, and a team of researchers created molecular "flasks," which are essentially self-assembling cage-like containers capable of housing other compounds inside them. These flasks may eventually allow researchers to isolate certain chemical reactions within or outside the flask.
The research is published in the July 22, 2011 issue of the journal Science.
"We wanted to create frameworks to serve as the 'hotel' for 'guest' molecules, which can deliver the function independent of framework design," said Ward. "This makes it possible to separate chemicals based on size or perform reactions inside well-defined cages, which could potentially give you more control over chemical reactivity and reaction products. Moreover, these frameworks may prove ideal for encapsulating a wide range of guest molecules, producing materials with new optical or magnetic properties."
The molecular flasks described by Ward and his collaborators take the shape of a truncated octahedron, one of 13 shapes described as an Archimedean solid, discovered by the Greek mathematician Archimedes. Archimedean solids are characterized by a specific number of sides that meet at corners which are all identical. The regularity of these shapes often means they are of particular interest to chemists and materials researchers looking to create complex materials that assemble themselves.
The extraordinary aspect of this work, supported by the National Science Foundation (NSF), is the self-assembly of the molecular tiles into a polyhedron, a well-defined, three-dimensional, geometric solid. The individual polyhedra assemble themselves using the attractive interactions associated with hydrogen bonds. They then further organize into a crystal lattice that resembles a porous structure called zeolite, an absorbent material with many industrial uses.
The new material differs from zeolite because it is constructed from organic building blocks rather than inorganic ones, which make it more versatile and easier to engineer. In general, inorganic compounds are considered mineral in origin, while organic compounds are considered biological in origin.
This discovery paves the way towards development of a new class of solids with properties that may prove useful for a range of industrial and consumer products.
"By using geometric design principles and very simple chemical precursors, the Ward group has been able to construct relatively sturdy materials which contain many identically sized and shaped cavities," explained Michael Scott, program director in the Division of Materials Research at NSF. "The hollow space inside these materials offers many exciting opportunities for chemists to do things such as isolate unstable molecules, catalyze unknown reactions and separate important chemical compounds."
Future research projects will try to create other types of Archimedean solids or use the truncated octahedron to house different types of functional molecules.
####
About National Science Foundation
The National Science Foundation (NSF) is an independent federal agency that supports fundamental research and education across all fields of science and engineering. In fiscal year (FY) 2011, its budget is about $6.9 billion. NSF funds reach all 50 states through grants to nearly 2,000 universities and institutions. Each year, NSF receives over 45,000 competitive requests for funding, and makes over 11,500 new funding awards. NSF also awards over $400 million in professional and service contracts yearly.
For more information, please click here
Contacts:
Media Contacts
Lisa Van Pay
NSF
(703) 292-8796
James Devitt
New York University
(212) 998-6808
Program Contacts
Linda S. Sapochak
NSF
(703) 292-4932
Principal Investigators
Michael D. Ward
New York University
(212) 998-8439
Copyright © National Science Foundation
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Molecular Nanotechnology
![]() Scientists push the boundaries of manipulating light at the submicroscopic level March 3rd, 2023
Scientists push the boundaries of manipulating light at the submicroscopic level March 3rd, 2023
![]() First electric nanomotor made from DNA material: Synthetic rotary motors at the nanoscale perform mechanical work July 22nd, 2022
First electric nanomotor made from DNA material: Synthetic rotary motors at the nanoscale perform mechanical work July 22nd, 2022
![]() Nanotech scientists create world's smallest origami bird March 17th, 2021
Nanotech scientists create world's smallest origami bird March 17th, 2021
Self Assembly
![]() Liquid crystal templated chiral nanomaterials October 14th, 2022
Liquid crystal templated chiral nanomaterials October 14th, 2022
![]() Nanoclusters self-organize into centimeter-scale hierarchical assemblies April 22nd, 2022
Nanoclusters self-organize into centimeter-scale hierarchical assemblies April 22nd, 2022
![]() Atom by atom: building precise smaller nanoparticles with templates March 4th, 2022
Atom by atom: building precise smaller nanoparticles with templates March 4th, 2022
![]() Nanostructures get complex with electron equivalents: Nanoparticles of two different sizes break away from symmetrical designs January 14th, 2022
Nanostructures get complex with electron equivalents: Nanoparticles of two different sizes break away from symmetrical designs January 14th, 2022
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








