Home > Press > Supramolecules get time to shine: Rice technique reveals interactions between nanotubes, photoluminescent materials
 |
| Rice University researchers have found a way to bind carbon nanotubes to a porous silicate particles to create supramolecules. The new material allows researchers to test interactions between nanotubes and photoluminescent materials. (Credit: Martí Lab/Rice University) |
Abstract:
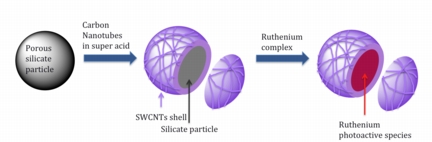
What looks like a spongy ball wrapped in strands of yarn -- but a lot smaller -- could be key to unlocking better methods for catalysis, artificial photosynthesis or splitting water into hydrogen, according to Rice University chemists who have created a platform to analyze interactions between carbon nanotubes and a wide range of photoluminescent materials.
Supramolecules get time to shine: Rice technique reveals interactions between nanotubes, photoluminescent materials
Houston, TX | Posted on July 12th, 2011The microscopic particles assembled in the lab of Angel Martí, an assistant professor of chemistry and bioengineering, combine single-walled carbon nanotubes with porous silicate materials that can absorb various molecules -- in this case, a ruthenium complex.
Martí, graduate student and lead author Avishek Saha and their colleagues reported their results today in the Royal Society of Chemistry journal Chemical Science.
The ability to immobilize individual carbon nanotubes on a solid surface is interesting enough, but combining supramolecular systems with nanomaterials to produce hybrids is unique, they said.
"This can be used as a general platform to study the interaction of not only ruthenium complexes, but most photoactive molecules can be encapsulated within these porous silicates in a very simple way without chemical modification, without anything," Marti said.
Saha endured trial and error at every step in bringing the new particles to fruition, first figuring out the best way to keep long, single-walled carbon nanotubes produced by the Rice-born HiPco process from aggregating into bundles while allowing them to adhere to the particles.
The solution suggested by co-author Matteo Pasquali, a Rice professor in chemical and biomolecular engineering and in chemistry, involved dissolving the bundles in chlorosulfonic acid, which added protons -- and thus a positive charge -- to each nanotube.
That was the key to making nanotubes attractive to the three types of silicate particles tested: a commercial version of MCM-41, a mesoporous material used as a molecular sieve; another version of MCM-41 synthesized at Rice by Saha, and microporous Zeolyte-Y.
"We don't fully understand the mechanism, but the truth is they have a very strong affinity to silicon oxide networks," said Marti, describing the nanotube-wrapped particles. "Once they're protonated, they just bind."
But that wasn't enough to create a proper platform because protonated nanoparticles are no longer photoluminescent, a quality the researchers required to "see" such tiny structures under a spectroscope. "Protonated nanotubes are cool, but we want to have pristine nanotubes," Martí said.
"We were stuck there for a while. We tried a lot of things," he said. Acetone, ammonia, chloroform and other substances would deprotonate the nanotubes, but would also release them from the silicate sponges and allow them to clump. But vinylpyrrolidone (VP) did the trick by giving the nanotubes a polymer-like coating while returning them to their pristine states.
"This becomes interesting not only from the standpoint of getting individualized nanotubes on top of a surface, but also because we got fluorescence of nanotubes not from a solution, but from a solid material," Martí said.
The experiment went one critical step further when the researchers introduced ruthenium molecules to the mix. The silicates absorbed the ruthenium molecules, putting them into close proximity with an array of nanotubes. Under a spectroscope, the ruthenium complexes would photoluminesce, but they saw something unexpected in the interaction.
"Basically, we found out that if you put a photoactive species (ruthenium) there and excite it with light, two different processes happen. If it has carbon nanotubes close by, it will transfer an electron to the nanotubes. There's a charge transfer, and we knew that would happen," Martí said. "What we didn't expect when we analyzed the spectrum was seeing two different species of ruthenium complexes, one with a very short photoluminescence lifetime and one very long."
The researchers theorized that ruthenium in the center of the sponge was too far from the nanotubes to transfer electrons, so it retained its standard luminescence.
The research leads to some interesting possibilities for materials science, Saha said. "MCM itself has many applications (as a mesoporous sieve in fuel refineries, for instance), and carbon nanotubes are wonderful materials that many people are interested in. We're just combining these two into a hybrid material that might have the virtues of both."
While pore sizes in zeolites are locked by their crystalline structure at 0.7 nanometers, pores in MCM can be customized, as Saha has done, to absorb specific materials. "There are many things we can do to tune the system that we haven't explored," he said; combining metal molecules or even quantum dots with MCM and nanotubes might lead to interesting results.
Martí said putting charged nanotubes on the surface of a solid also opens the door to use them as catalysts in solar-energy conversion. "You need that driving force, that charge separation, for artificial photosynthesis," he said.
Co-authors of the paper are Rice graduate students Saunab Ghosh and Natnael Behabtu.
The Welch Foundation supported the research.
####
About Rice University
Located on a 285-acre forested campus in Houston, Texas, Rice University is consistently ranked among the nation's top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is known for its “unconventional wisdom." With 3,485 undergraduates and 2,275 graduate students, Rice's undergraduate student-to-faculty ratio is less than 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice has been ranked No. 1 for best quality of life multiple times by the Princeton Review and No. 4 for "best value" among private universities by Kiplinger's Personal Finance. To read "What they're saying about Rice," go to futureowls.rice.edu/images/futureowls/Rice_Brag_Sheet.pdf.
For more information, please click here
Contacts:
David Ruth
713-348-6327
Mike Williams
713-348-6728
Copyright © Rice University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Imaging
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
![]() Observation of left and right at nanoscale with optical force October 6th, 2023
Observation of left and right at nanoscale with optical force October 6th, 2023
Nanotubes/Buckyballs/Fullerenes/Nanorods/Nanostrings
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
![]() Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Solar/Photovoltaic
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() Charged “molecular beasts” the basis for new compounds: Researchers at Leipzig University use “aggressive” fragments of molecular ions for chemical synthesis November 3rd, 2023
Charged “molecular beasts” the basis for new compounds: Researchers at Leipzig University use “aggressive” fragments of molecular ions for chemical synthesis November 3rd, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








