Home > Press > The Heat Is on for Sodium-Manganese Oxide Rechargeable Batteries: New method to make sodium ion-based battery cells could lead to better, cheaper batteries for the electrical grid
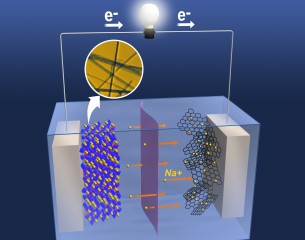 |
| The uniform nanostructure of heat-treated manganese oxide provides tunnels for sodium ions to flow through, improving the performance of the electrodes. |
Abstract:
By adding the right amount of heat, researchers have developed a method that improves the electrical capacity and recharging lifetime of sodium ion rechargeable batteries, which could be a cheaper alternative for large-scale uses such as storing energy on the electrical grid.
The Heat Is on for Sodium-Manganese Oxide Rechargeable Batteries: New method to make sodium ion-based battery cells could lead to better, cheaper batteries for the electrical grid
Richland, WA | Posted on June 7th, 2011To connect solar and wind energy sources to the electrical grid, grid managers require batteries that can store large amounts of energy created at the source. Lithium ion rechargeable batteries -- common in consumer electronics and electric vehicles -- perform well, but are too expensive for widespread use on the grid because many batteries will be needed, and they will likely need to be large. Sodium is the next best choice, but the sodium-sulfur batteries currently in use run at temperatures above 300 degrees Celsius, or three times the temperature of boiling water, making them less energy efficient and safe than batteries that run at ambient temperatures.
Battery developers want the best of both worlds -- to use both inexpensive sodium and use the type of electrodes found in lithium rechargeables. A team of scientists at the Department of Energy's Pacific Northwest National Laboratory and visiting researchers from Wuhan University in Wuhan, China used nanomaterials to make electrodes that can work with sodium, they reported June 3 online in the journal Advanced Materials.
"The sodium-ion battery works at room temperature and uses sodium ions, an ingredient in cooking salt. So it will be much cheaper and safer," said PNNL chemist Jun Liu, who co-led the study with Wuhan University chemist Yuliang Cao.
The electrodes in lithium rechargeables that interest researchers are made of manganese oxide. The atoms in this metal oxide form many holes and tunnels that lithium ions travel through when batteries are being charged or are in use. The free movement of lithium ions allows the battery to hold electricity or release it in a current. But simply replacing the lithium ions with sodium ions is problematic -- sodium ions are 70 percent bigger than lithium ions and don't fit in the crevices as well.
To find a way to make bigger holes in the manganese oxide, PNNL researchers went much much smaller. They turned to nanomaterials -- materials made on the nanometer-sized scale, or about a million times thinner than a dime -- that have surprising properties due to their smallness. For example, the short distances that sodium ions have to travel in nanowires might make the manganese oxide a better electrode in ways unrelated to the size of the tunnels..
To explore, the team mixed two different kinds of manganese oxide atomic building blocks -- one whose atoms arrange themselves in pyramids, and another one whose atoms form an octahedron, a diamond-like structure from two pyramids stuck together at their bases. They expected the final material to have large S-shaped tunnels and smaller five-sided tunnels through which the ions could flow.
After mixing, the team treated the materials with temperatures ranging from 450 to 900 degrees Celsius, then examined the materials and tested which treatment worked best. Using a scanning electron microscope, the team found that different temperatures created material of different quality. Treating the manganese oxide at 750 degrees Celsius created the best crystals: too low and the crystals appeared flakey, too high and the crystals turned into larger flat plates.
Zooming in even more using a transmission electron microscope at EMSL, DOE's Environmental Molecular Sciences Laboratory on PNNL's campus, the team saw that manganese oxide heated to 600 degrees had pockmarks in the nanowires that could impede the sodium ions, but the 750 degree-treated wires looked uniform and very crystalline.
But even the best-looking material is just window-dressing if it doesn't perform well. To find out if it lived up to its good looks, the PNNL-Wuhan team dipped the electrode material in electrolyte, the liquid containing sodium ions that will help the manganese oxide electrodes form a current. Then they charged and discharged the experimental battery cells repeatedly.
The team measured peak capacity at 128 milliAmp hours per gram of electrode material as the experimental battery cell discharged. This result surpassed earlier ones taken by other researchers, one of which achieved peak capacity of 80 milliAmp hours per gram for electrodes made from manganese oxide but with a different production method. The researchers think the lower capacity is due to sodium ions causing structural changes in that manganese oxide that do not occur or occur less frequently in the heat-treated nano-sized material.
In addition to high capacity, the material held up well to cycles of charging and discharging, as would occur in consumer use. Again, the material treated at 750 Celsius performed the best: after 100 cycles of charging-discharging, it lost only 7 percent of its capacity. Material treated at 600 Celsius or 900 Celsius lost about 37 percent and 25 percent, respectively.
Even after 1,000 cycles, the capacity of the 750 Celsius-treated electrodes only dropped about 23 percent. The researchers thought the material performed very well, retaining 77 percent of its initial capacity.
Last, the team charged the experimental cell at different speeds to determine how quickly it could take up electricity. The team found that the faster they charged it, the less electricity it could hold. This suggested to the team that the speed with which sodium ions could diffuse into the manganese oxide limited the battery cell's capacity -- when charged fast, the sodium ions couldn't enter the tunnels fast enough to fill them up.
To compensate for the slow sodium ions, the researchers suggest in the future they make even smaller nanowires to speed up charging and discharging. Grid batteries need fast charging so they can collect as much newly made energy coming from renewable sources as possible. And they need to discharge fast when demands shoots up as consumers turn on their air conditioners and television sets, and plug in their electric vehicles at home.
Such high performing batteries could take the heat off an already taxed electrical power grid.
This work was funded by the Department of Energy's Office of Science and Office of Electricity Delivery & Energy Reliability.
####
About Pacific Northwest National Laboratory
Pacific Northwest National Laboratory is a Department of Energy Office of Science national laboratory where interdisciplinary teams advance science and technology and deliver solutions to America's most intractable problems in energy, national security and the environment. PNNL employs 4,900 staff, has an annual budget of nearly $1.1 billion, and has been managed by Ohio-based Battelle since the lab's inception in 1965. Follow PNNL on Facebook, LinkedIn and Twitter.
EMSL, the Environmental Molecular Sciences Laboratory located at Pacific Northwest National Laboratory, is a national scientific user facility sponsored by the Department of Energy's Office of Science. EMSL offers an open, collaborative environment for scientific discovery to researchers around the world. EMSL’s technical experts and suite of custom and advanced instruments are unmatched. Its integrated computational and experimental capabilities enable researchers to realize fundamental scientific insights and create new technologies.
For more information, please click here
Contacts:
Mary Beckman
(509) 375-3688
Copyright © Newswise
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
![]() Three-pronged approach discerns qualities of quantum spin liquids November 17th, 2023
Three-pronged approach discerns qualities of quantum spin liquids November 17th, 2023
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Nanoelectronics
![]() Interdisciplinary: Rice team tackles the future of semiconductors Multiferroics could be the key to ultralow-energy computing October 6th, 2023
Interdisciplinary: Rice team tackles the future of semiconductors Multiferroics could be the key to ultralow-energy computing October 6th, 2023
![]() Key element for a scalable quantum computer: Physicists from Forschungszentrum Jülich and RWTH Aachen University demonstrate electron transport on a quantum chip September 23rd, 2022
Key element for a scalable quantum computer: Physicists from Forschungszentrum Jülich and RWTH Aachen University demonstrate electron transport on a quantum chip September 23rd, 2022
![]() Reduced power consumption in semiconductor devices September 23rd, 2022
Reduced power consumption in semiconductor devices September 23rd, 2022
![]() Atomic level deposition to extend Moore’s law and beyond July 15th, 2022
Atomic level deposition to extend Moore’s law and beyond July 15th, 2022
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








