Home > Press > Scientists uncover chemical transformations in cobalt nanoparticles
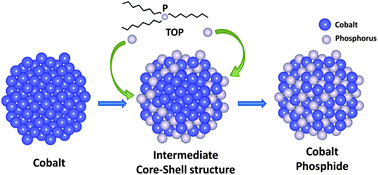 |
| Provided/Robinson lab The evolution schematics of transition from cobalt to cobalt phosphide nanocrystals. |
Abstract:
Understanding the intricacies of how nanoparticles undergo chemical transformations could lead to better ways to tailor their composition, which can lead to advanced material properties.
Scientists uncover chemical transformations in cobalt nanoparticles
Ithaca, NY | Posted on May 24th, 2011Using the Cornell High Energy Synchrotron Source, scientists led by Richard Robinson, assistant professor of materials science and engineering, uncovered exactly what happens when cobalt nanoparticles transform into two phases of cobalt phosphides.
Their work, published in the Journal of Materials Chemistry, was featured by the journal as a "Hot Article" earlier this month.
The effect Robinson's team observed in the cobalt phosphide transitions was a nanoparticle hollowing due to asymmetric diffusivities of cations and anions. In other words, the cations move out from the core faster than anions can diffuse in, leading to a hollow particle.
Other groups have reported on this "Kirkendall" effect, but the Robinson team was the first to show that this hollowing is more complex than previously thought and can be studied as a two-step process. Their work could be used to control this process and produce complex particles with properties tailored for use in energy applications. Metal phosphides have a wide range of properties -- ferromagnetism, superconductivity, catalytic activity and magnetoresistance among them.
The work was done in collaboration with scientists led by Richard Hennig, assistant professor of materials science and engineering. It was supported by King Abdullah University of Science and Technology, the Cornell Center for Materials Research and the Energy Materials Center at Cornell.
####
For more information, please click here
Contacts:
Media Contact:
Blaine Friedlander
(607) 254-8093
Cornell Chronicle:
Anne Ju
(607) 255-9735
Copyright © Cornell University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Materials/Metamaterials/Magnetoresistance
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Research partnerships
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() 'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








