Home > Press > Nano-Motor with a Light Switch Light-triggered myosin allows real-time study of cells
 |
Abstract:
Molecular "motors" are at the root of most biological movement. They propel cell components, whole cells, and even our muscles on command. Barbara Imperiali and a team from the Massachusetts Institute of Technology (Cambridge, USA), the University of Virginia (Charlottesville, USA), and the National Institutes of Health (USA) have now provided the motor protein myosin with an "on switch" that is activated by light. As the scientists report in the journal Angewandte Chemie, this should make it possible to follow cellular processes that involve myosin in real time.
Nano-Motor with a Light Switch Light-triggered myosin allows real-time study of cells
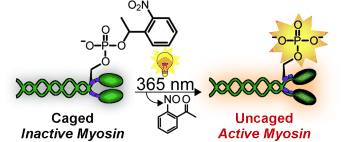
Cambridge, MA and Charlottesville, VA and Bethesda, MD | Posted on May 10th, 2011In order for our muscles to contract, two types of fibrous proteins, myosin and actin, must interact. Driven by splitting of the cellular fuel adenosine triphosphate (ATP), "buttons" on the myosin molecules attach, allowing the myosin to dangle off of the actin filaments. In non-muscular cells, myosin ensures that the cell constricts itself in the division process. Myosin consists of several different protein chains. The activity of non-muscular myosin is regulated through its regulatory light chain. As soon as a phosphate group binds to a specific site (Ser19) of the light chain (phosphorylation), myosin become active. The activity can be amplified through binding of a second phosphate group at a neighboring site (Thr18).
Myosin has been intensively studied. However, it has not been possible to examine precisely what happens after activation of the molecule in living cells both spatially and over time. This research team has now found a trick that makes real-time observations possible: A myosin molecule that can be switched on by light. To achieve this, the researchers used protein synthesis to produce a synthetic regulatory chain that already contains one or two phosphate groups. The trick is that one of the phosphate groups is covered by a cage. In this form, the chain is inactive. Irradiation with light makes the cage split off, switching on the regulatory chain and activating the myosin.
The researchers replaced the natural light chain in myosin molecules with their synthetic one and introduced this light-activated myosin into cells. Irradiation activates it at a defined time in a defined place. In this way, the researchers hope to observe what happens after the activation of myosin in a cell in real time.
####
For more information, please click here
Contacts:
Barbara Imperiali
Massachusetts Institute of Technology
Cambridge (USA)
Tel: (617) 253-1838
Fax: (617) 452 2419
Copyright © Angewandte Chemie
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() Angewandte Chemie International Edition ,
Angewandte Chemie International Edition ,
| Related News Press |
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








