Home > Press > On the Way to Hydrogen Storage? A magnesium hydride cluster as a model for a hydrogen storage material at the sub-nanometer level
 |
Abstract:
The car of the future could be propelled by a fuel cell powered with hydrogen. But what will the fuel tank look like? Hydrogen gas is not only explosive but also very space-consuming. Storage in the form of very dense solid metal hydrides is a particularly safe alternative that accommodates the gas in a manageable volume. As the storage tank should also not be too heavy and expensive, solid-state chemists worldwide focus on hydrides containing light and abundant metals like magnesium. Sjoerd Harder and his co-workers at the Universities of Groningen (Netherlands) and Duisburg-Essen (Germany) now take the molecular approach. As the researchers report in the journal Angewandte Chemie, extremely small clusters of molecular magnesium hydride could be a useful model substance for more precise studies about the processes involved in hydrogen storage.
On the Way to Hydrogen Storage? A magnesium hydride cluster as a model for a hydrogen storage material at the sub-nanometer level
Germany | Posted on April 19th, 2011Magnesium hydride (MgH2) can release hydrogen when needed and the resulting magnesium metal reacts back again to form the hydride by pressurizing with hydrogen at a "gas station". Unfortunately, this is an idealized picture. Not only is the speed of hydrogen release/uptake excessively slow (kinetics) but it also only operates at higher temperatures (thermodynamics). The hydrides, the negatively charged hydrogen atoms (H─), are bound so strongly in the crystal lattice of magnesium cations (Mg2+) that temperatures of more than 300 ˚C are needed to release the hydrogen gas.
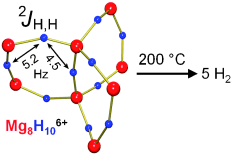
Particularly intensive milling has made it possible to obtain nanocrystalline materials, which, on account of its larger surface, rapidly release or take up hydrogen. However, the high stability of the magnesium hydride still translates to rather high release temperatures. According to recent computer calculations, magnesium hydride clusters of only a few atoms possibly could generate hydrogen at temperatures far below 300 °C. Clusters with less than 20 Mg2+ ions are smaller than one nanometer and behave differently from the bulk material. Their hydride ions have fewer Mg2+ neighbors and are more weakly bound. However, it is extremely difficult to obtain such tiny clusters by milling. In Harder's "bottom-up" approach, magnesium hydride clusters are made by starting from molecules. The challenge is to prevent such clusters from forming very stable bulk material. Using a special ligand system, they could trap a cluster that resembles a paddle wheel made of eight Mg2+ and ten H─ ions. For the first time it was shown that molecular clusters indeed release hydrogen already at the temperature of 200 °C.
This largest magnesium hydride cluster reported to date is not practical for efficient hydrogen storage but shines new light on a current problem. It is easily studied by molecular methods and as a model system could provide detailed insights in hydrogen storage.
####
For more information, please click here
Contacts:
Sjoerd Harder
University of Groningen (Netherlands)
H. H. (Hilda) Biemold +31 50 363 4235
Postal address Stratingh Institute for Chemistry
University of Groningen
Nijenborgh 4
NL-9747 AG Groningen
The Netherlands
E-mail
+31-50-363 4322
FAX +31 50 363 4296
Copyright © Wiley-VCH Verlag GmbH & Co. KGaA
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








