Home > Press > Berkeley Lab Researchers Report Tandem Catalysis in Nanocrystal Interfaces: Could be a Boon to Green Energy
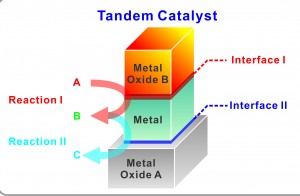 |
| In a unique new bilayer nanocatalyst system, single layers of metal and metal oxide nanocubes are deposited to create two distinct metal–metal oxide interfaces that allow for multiple, sequential catalytic reactions to be carried out selectively and in tandem. (Image courtesy of Yang group) |
Abstract:
In a development that holds intriguing possibilities for the future of industrial catalysis, as well as for such promising clean green energy technologies as artificial photosynthesis, researchers with the U.S. Department of Energy (DOE)'s Lawrence Berkeley National Laboratory (Berkeley Lab) have created bilayered nanocrystals of ametal-metal oxide that are the first to feature multiple catalytic sites on nanocrystal interfaces. These multiple catalytic sites allow for multiple, sequential catalytic reactions to be carried out selectively and in tandem.
Berkeley Lab Researchers Report Tandem Catalysis in Nanocrystal Interfaces: Could be a Boon to Green Energy
Berkeley, CA | Posted on April 14th, 2011"The demonstration of rationally designed and assembled nanocrystal bilayers with multiple built-in metal-metal oxide interfaces for tandem catalysis represents a powerful new approach towards designing high-performance, multifunctional nanostructured catalysts for multiple-step chemical reactions," says the leader of this research Peidong Yang, a chemist who holds joint appointments with Berkeley Lab's Materials Sciences Division, and the University of California Berkeley's Chemistry Department and Department of Materials Science and Engineering.
Yang is the corresponding author of a paper describing this research that appears in the journal Nature Chemistry. The paper is titled "Nanocrystal bilayer for tandem catalysis." Co-authoring the paper were Yusuke Yamada, Chia-Kuang Tsung, Wenyu Huang, Ziyang Huo, Susan Habas, Tetsuro Soejima, Cesar Aliaga and leading authority on catalysis Gabor Somorjai.
Catalysts - substances that speed up the rates of chemical reactions without themselves being chemically changed - are used to initiate virtually every industrial manufacturing process that involves chemistry. Metal catalysts have been the traditional workhorses, but in recent years, with the advent of nano-sized catalysts, metal,oxide and their interface have surged in importance.
"High-performance metal-oxide nanocatalysts are central to the development of new-generation energy conversion and storage technologies," Yang says. "However, to significantly improve our capability of designing better catalysts, new concepts for the rational design and assembly of metal-metal oxide interfaces are needed."
Studies in recent years have shown that for nanocrystals, the size and shape - specifically surface faceting with well-defined atomic arrangements - can have an enormous impact on catalytic properties. This makes it easier to optimize nanocrystal catalysts for activity and selectivity than bulk-sized catalysts. Shape- and size-controlled metal oxide nanocrystal catalysts have shown particular promise.
"It is well-known that catalysis can be modulated by using different metal oxide supports, or metal oxide supports with different crystal surfaces," Yang says. "Precise selection and control of metal-metal oxide interfaces in nanocrystals should therefore yield better activity and selectivity for a desired reaction."
To determine whether the integration of two types of metal oxide interfaces on the surface of a single active metal nanocrystal could yield a novel tandem catalyst for multistep reactions, Yang and his coauthors used the Langmuir-Blodgett assembly technique to deposit nanocube monolayers of platinum and cerium oxide on a silica (silicon dioxide) substrate. The nanocube layers were each less than 10 nanometers thick and stacked one on top of the other to create two distinct metal-metal oxide interfaces - platinum-silica and cerium oxide-platinum. These two interfaces were then used to catalyze two separate and sequential reactions. First, the cerium oxide-platinum interface catalyzed methanol to produce carbon monoxide and hydrogen. These products then underwent ethylene hydroformylation through a reaction catalyzed by the platinum-silica interface. The final result of this tandem catalysis was propanal.
"The cubic shape of the nanocrystal layers is ideal for assembling metal-metal oxide interfaces with large contact areas," Yang says. "Integrating binary nanocrystals to form highly ordered superlattices is a new and highly effective way to form multiple interfaces with new functionalities."
Yang says that the concept of tandem catalysis through multiple interface design that he and his co-authors have developed should be especially valuable for applications in which multiple sequential reactions are required to produce chemicals in a highly active and selective manner. A prime example is artificial photosynthesis, the effort to capture energy from the sun and transform it into electricity or chemical fuels. To this end, Yang leads the Berkeley component of the Joint Center for Artificial Photosynthesis, a new Energy Innovation Hub created by the U.S. Department of Energy that partners Berkeley Lab with the California Institute of Technology (Caltech).
"Artificial photosynthesis typically involves multiple chemical reactions in a sequential manner, including, for example, water reduction and oxidation, and carbon dioxide reduction," says Yang. "Our tandem catalysis approach should also be relevant to photoelectrochemical reactions, such as solar water splitting, again where sequential, multiple reaction steps are necessary. For this, however, we will need to explore new metal oxide or other semiconductor supports, such as titanium dioxide, in our catalyst design."
This research was supported by the DOE Office of Science.
####
About Berkeley Lab
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 12 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
For more information, please click here
Contacts:
Lynn Yarris
(510) 486-5375
Copyright © Berkeley Lab
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() For more about the research of Peidong Yang and his group, visit the Website at
For more about the research of Peidong Yang and his group, visit the Website at
![]() For more information about the Joint Center for Artificial Photosynthesis visit the Website at
For more information about the Joint Center for Artificial Photosynthesis visit the Website at
![]() For more information about the research of catalysis authority Gabor Somorjai, visit the Website at
For more information about the research of catalysis authority Gabor Somorjai, visit the Website at
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
![]() Three-pronged approach discerns qualities of quantum spin liquids November 17th, 2023
Three-pronged approach discerns qualities of quantum spin liquids November 17th, 2023
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Solar/Photovoltaic
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() Charged “molecular beasts” the basis for new compounds: Researchers at Leipzig University use “aggressive” fragments of molecular ions for chemical synthesis November 3rd, 2023
Charged “molecular beasts” the basis for new compounds: Researchers at Leipzig University use “aggressive” fragments of molecular ions for chemical synthesis November 3rd, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








