Home > Press > Future Fuels for Everyone Powered by the Sun
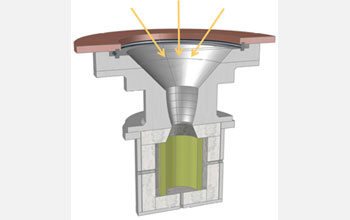 |
| The ETH-Caltech solar reactor for producing hydrogen and carbon monoxide from water and carbon dioxide via the two-step thermochemical cycle with ceria.
Credit: California Institute of Technology |
Abstract:
"At the California Institute of Technology, they're developing a way to turn sunlight and water into fuel for our cars."--President Barack Obama, Jan. 25, 2011
The Sun is Earth's primary energy source and harnessing its abundant light is the Holy Grail of renewable energy
Now, a group of scientists has demonstrated a new way to use sunlight, water (H2O) and carbon dioxide (CO2)--some of the cheapest and most commonplace stuff on Earth--to make unlimited amounts of fuel to power almost anything, anywhere.
Future Fuels for Everyone Powered by the Sun
Arlington, VA | Posted on April 6th, 2011The method uses concentrated heat from the sun to convert water and carbon dioxide into hydrogen (H2) or carbon monoxide (CO). Large amounts of these two gases could be combined to make liquid fuel that fits into America's existing energy economy.
"Alternatively, you could use the H2 and CO to make methane (natural gas) for a gas-fired electricity generator," said Sossina Haile, professor of Materials Science and of Chemical Engineering at California Institute of Technology in Pasadena. "Or, because the fuels we produce are so pure, they could be easily used to run fuel cells, which generate power very efficiently."
The researchers say one of the most exciting things about the discovery is its versatility. "We are not dictating to the user what the energy infrastructure should be," Haile said. "We are making solar energy easy to use by putting it into a form that our industry is used to seeing and making it available on demand."
Doing the two-step
Scientists have long known how to convert water and carbon dioxide into hydrogen and carbon monoxide. But to do it cheaply and efficiently enough to make the process affordable on a wide scale has been the issue. Part of the problem was the need for expensive and rare elements, such as platinum or iridium, to act as catalysts that encourage the conversion to happen.
So Haile and her team took a novel approach; they tried ceria, a material used in the walls of self-cleaning ovens. Ceria is the oxidized or "rust" form of the element cerium, which is more abundant, and therefore cheaper, than other metals that could do the same job.
The new method requires two steps, the first at high temperature using concentrated heat from the sun (about 3,000 degrees Fahrenheit), and the second at a much lower temperature.
Haile describes the process this way, "If we heat ceria up, the material 'naturally' releases some oxygen from its structure. If we then cool it back down, those oxygen vacancies want to be refilled. In other words, the ceria 'exhales' oxygen at high temperature and then 'inhales' it back when the temperature is lowered."
To make fuel, the second step requires the presence of water and carbon dioxide gases. "At lower temperatures, the cerium, the hydrogen and the carbon all want the oxygen, but the cerium wants it most," Haile said. "So the oxygen vacancies in the ceria are filled by stripping oxygen from H2O and CO2, leaving H2 and CO."
An international collaboration
Haile and her Caltech team, supported by an award from the National Science Foundation, recently published a paper describing the breakthrough in the journal Science. For this project, they collaborated with researchers led by Aldo Steinfeld, a renewable energy technology professor at the Swiss Federal Institute of Technology, also called ETH Zürich, in Switzerland. Steinfeld also leads the Solar Technology Laboratory at the Paul Scherrer Institute in Switzerland.
Two pieces of equipment were needed for the experiment. The first piece, built at Caltech, is a reactor "just a bit smaller than a gallon-jug," Haile said. The reactor is basically a cylindrical container lined with ceria that has input and output lines for the gases.
The second piece is a solar concentrator, which is the most difficult part to build. The concentrator is basically a set of giant curved mirrors that gather sunlight from a wide area. For this experiment, the researchers were able to use an existing solar concentrator located at the Paul Scherrer Institute.
The Caltech scientists took their reactor to Switzerland and attached it to the bottom of the concentrator, allowing the sunlight to heat up the ceria inside. Then they piped steam and carbon dioxide into the reactor and measured the hydrogen and carbon monoxide gases flowing out.
Cheaper and more efficient
How far reaching could this new technology be and how much oil, gas or coal could it replace?
"The abundance of cerium means that this approach could have a significant impact on our national energy budget," Haile said. Because cerium is 100,000 times more abundant than the precious metal platinum, she said, the cost would be many orders of magnitude smaller.
For this experiment, the efficiency of the reactor at converting sunlight to usable energy measured just under one percent, which Haile said is comparable to other methods. However, this was a first cut, aimed at simply proving that the process is practical and could be done economically.
Before bringing the technology to market, Haile said, the reactor design needs to be much tighter to get better efficiency.
"As a second step, it will be important to develop materials with even better characteristics than ceria," she added.
"Ideally, one wants a material with a smaller temperature swing required as this will also increase efficiency," Haile said. "In addition, if both the high and low temperatures can be lowered, the overall system lifetime will be improved. Better materials could result in a better process."
-- Holly Bigelow Martin
Investigators
Roy Smith
Carlos Levi
Dorothy Pak
Sossina Haile
Tresa Pollock
Nicola Spaldin
Michael Chabinyc
Christian Van de Walle
Related Institutions/Organizations
California Institute of Technology
University of California-Santa Barbara
####
For more information, please click here
Contacts:
4201 Wilson Boulevard
Arlington, Virginia 22230, USA
Tel: (703) 292-5111
FIRS: (800) 877-8339
Copyright © National Science Foundation
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Solar/Photovoltaic
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() Charged “molecular beasts” the basis for new compounds: Researchers at Leipzig University use “aggressive” fragments of molecular ions for chemical synthesis November 3rd, 2023
Charged “molecular beasts” the basis for new compounds: Researchers at Leipzig University use “aggressive” fragments of molecular ions for chemical synthesis November 3rd, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








