Home > Press > Tuning graphene film so it sheds water
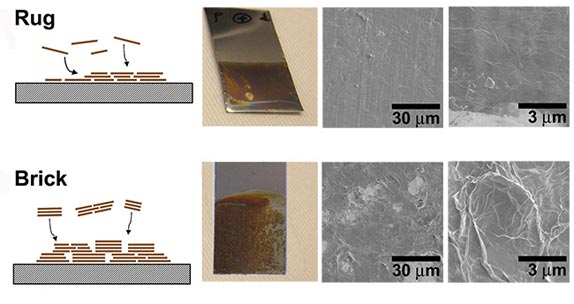 |
| Dickerson can tweak the process for creating films of graphene oxide so they are formed by "rug" process, above, that is extreme smooth and "water loving" or by a "brick" process, below, that is rough and "water hating." (Image courtesy of James Dickerson) |
Abstract:
Windshields that shed water so effectively that they don't need wipers. Ship hulls so slippery that they glide through the water more efficiently than ordinary hulls.
These are some of the potential applications for graphene, one of the hottest new materials in the field of nanotechnology, raised by the research of James Dickerson, assistant professor of physics at Vanderbilt.
Tuning graphene film so it sheds water
Nashville, TN | Posted on February 2nd, 2011Dickerson and his colleagues have figured out how to create a freestanding film of graphene oxide and alter its surface roughness so that it either causes water to bead up and run off or causes it to spread out in a thin layer.
"Graphene films are transparent and, because they are made of carbon, they are very inexpensive to make," Dickerson said. "The technique that we use can be rapidly scaled up to produce it in commercial quantities."
His approach is documented in an article published online by the journal ACSNano on Nov. 26.
Graphene is made up of sheets of carbon atoms arranged in rings - something like molecular chicken wire. Not only is this one of the thinnest materials possible, but it is 10 times stronger than steel and conducts electricity better at room temperature than any other known material. Graphene's exotic properties have attracted widespread scientific interest, but Dickerson is one of the first to investigate how it interacts with water.
Many scientists studying graphene make it using a dry method, called "mechanical cleavage," that involves rubbing or scraping graphite against a hard surface. The technique produces sheets that are both extremely thin and extremely fragile. Dickerson's method can produce sheets equally as thin but considerable stronger than those made by other techniques. It is already used commercially to produce a variety of different coatings and ceramics. Known as electrophoretic deposition, this "wet" technique combines an electric field within a liquid medium to create nanoparticle films that can be transferred to another surface.
Dickerson and his colleagues found that they could change the manner in which the graphene oxide particles assemble into a film by varying the pH of the liquid medium and the electric voltage used in the process. One pair of settings lay down the particles in a "rug" arrangement that creates a nearly atomically smooth surface. A different pair of settings causes the particles to clump into tiny "bricks" forming a bumpy and uneven surface. The researchers determined that the rug surface causes water to spread out in a thin layer, while the brick surface causes water to bead up and run off.
Dickerson is pursuing an approach that could create film that enhances these water-associated properties, making them even more effective at either spreading out water or causing it to bead up and run off. There is considerable academic and commercial interest in the development of coatings with these enhanced properties, called super-hydrophobic and super-hydrophilic. Potential applications range from self-cleaning glasses and clothes to antifogging surfaces to corrosion protection and snow-load protection on buildings. However, effective, low-cost and durable coatings have yet to make it out of the laboratory.
Dickerson's idea is to apply his basic procedure to "fluorographene" - a fluorinated version of graphene that is a two-dimensional version of Teflon - recently produced by Kostya S. Novoselov and Andre K. Geim at the University of Manchester, who received the 2010 Nobel Prize for the discovery of graphene. Normal fluorographene under tension should be considerably more effective in repelling water than graphene oxide. So there is a good chance a "brick" version and a "rug" version would have extreme water-associated effects, Dickerson figures.
Graduate students Saad Hasan, John Rigueur, Robert Harl and Alex Krejci, postdoctoral research scientist Isabel Gonzalo-Juan and Associate Professor of Chemical and Biomolecular Engineering Bridget R. Rogers contributed to the research, which was funded by a Vanderbilt Discovery grant and by the National Science Foundation.
(Hat Tip to Ron at graphene-info.com http://www.graphene-info.com/graphene-can-be-made-repel-water-very-effectively )
####
For more information, please click here
Contacts:
David Salisbury
(615) 322-NEWS
Copyright © Vanderbilt University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Marine/Watercraft
![]() Strain-sensing smart skin ready to deploy: Nanotube-embedded coating detects threats from wear and tear in large structures July 15th, 2022
Strain-sensing smart skin ready to deploy: Nanotube-embedded coating detects threats from wear and tear in large structures July 15th, 2022
![]() A sunlight-driven “self-healing” anti-corrosion coating May 27th, 2022
A sunlight-driven “self-healing” anti-corrosion coating May 27th, 2022
![]() Quantum tech in space? Scientists design remote monitoring system for inaccessible quantum devices February 11th, 2022
Quantum tech in space? Scientists design remote monitoring system for inaccessible quantum devices February 11th, 2022
![]() Expanding the freedom of design: powder coating on FRP thanks to conductive gelcoats with graphene nanotubes March 3rd, 2021
Expanding the freedom of design: powder coating on FRP thanks to conductive gelcoats with graphene nanotubes March 3rd, 2021
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Academic/Education
![]() Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
![]() Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Nanotubes/Buckyballs/Fullerenes/Nanorods/Nanostrings
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
![]() Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Automotive/Transportation
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








