Home > Press > Charging makes nano-sized electrodes swell, elongate and spiral
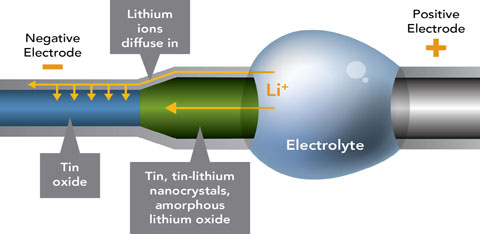 |
| This nano-sized battery reveals how positive lithium ions flood the negative electrode (blue), changing the size, shape and nature of the material (the green part of the electrode). Some rechargeable materials might be more resilient than others to the repeated shape-changing. Credit Pacific Northwest National Laboratory |
Abstract:
High-resolution video shows how batteries wear out over time
Charging makes nano-sized electrodes swell, elongate and spiral
Richland, WA | Posted on December 14th, 2010New high resolution images of electrode wires made from materials used in rechargeable lithium ion batteries shows them contorting as they become charged with electricity. The thin, nano-sized wires writhe and fatten as lithium ions flow in during charging, according to a paper in this week's issue of the journal Science. The work suggests how rechargeable batteries eventually give out and might offer insights for building better batteries.
Battery developers know that recharging and using lithium batteries over and over damages the electrode materials, but these images at nanometer scale offer a real-life glimpse into how. Thin wires of tin oxide, which serve as the negative electrode, fatten by a third and stretch twice as long due to lithium ions coursing in. In addition, the lithium ions change the tin oxide from a neatly arranged crystal to an amorphous glassy material.
"Nanowires of tin oxide were able to withstand the deformations associated with electrical flow better than bulk tin oxide, which is a brittle ceramic," said Chongmin Wang, a materials scientist at the Department of Energy's Pacific Northwest National Laboratory. "It reminds me of making a rope from steel — you wind together thinner wires rather than making one thick rope."
In one of the videos (*) the nanowire appears like a straw, while the lithium ions seem like a beverage being sucked up through it. Repeated shape changes could damage the electrode materials by introducing tiny defects that accumulate over time.
Chasing Electrons
In previous work at DOE's Environmental Molecular Sciences Laboratory on the PNNL campus, Wang, PNNL chemist Wu Xu and other colleagues succeeded in taking a snapshot of a larger nanowire of about one micrometer — or one-hundredth the width of a human hair — that had been partially charged. But the experimental set-up didn't show charging in action.
To view the dynamics of an electrode being charged, Wang and Xu teamed up with Jianyu Huang at DOE's Center for Integrated Nanotechnologies at Sandia National Laboratories in New Mexico and others. The team used a specially outfitted transmission electron microscope to set up a miniature battery. This instrument allowed them to image smaller wires of about 200 nanometers in diameter (about a fifth the width of the previous nanowires) while charging it.
Rechargeable lithium ion batteries work because lithium ions love electrons. Positively charged lithium ions normally hang out in the positive electrode, where a metal oxide shares its electrons with lithium. But charging a battery pumps free electrons into the negative electrode, which sits across a lake of electrolytes through which lithium ions can swim but electrons can't. The lithium desires the electrons on the negative side of the lake more than the electrons it shares with the metal oxide on the positive side. So lithium ions flow from the positive to the negative electrode, pairing up with free electrons there.
But electrons are fickle. Using a battery in a device allows the electrons to slip out of the negative electrode, leaving the lithium ions behind. So without free electron companions, the lithium ions return to the positive electrode and the metal oxide's embrace.
Wang's miniature battery included a positive electrode of lithium cobalt oxide and a negative electrode made from thin nanowires of tin oxide. Between the two electrodes, an electrolyte provided a conduit for lithium ions and a barrier for electrons. The electrolyte was specially designed to withstand the conditions in the microscope.
When the team charged the miniature battery at a constant voltage, lithium ions wicked up through the tin oxide wire, drawn by the electrons at the negative electrode. The wire fattened and lengthened by about 250 percent in total volume, and twisted like a snake.
In addition, the microscopy showed that the wire started out in a crystalline form. But the lithium ions changed the tin oxide to a glassy material, in which atoms are arranged more randomly than in a crystal. The researchers concluded the amount of deformation occurring during charging and use might wear down battery materials after a while. Even so, the tin oxide appeared to fare better as a nanowire than in its larger, bulk form.
"We think this work will stimulate new thinking for energy storage in general," said Wang. "This is just the beginning, and we hope with continued work it will show us how to design a better battery."
Future work will include imaging what happens when such a miniature battery is repeatedly charged and discharged. When a battery gets used, the lithium ions must run back through the tin oxide wire and across the electrolyte to the positive electrode. How much structural damage the receding lithium leaves in its wake will help researchers understand why rechargeable batteries stop working after being recharged so many times.
The researchers would also like to develop a fully functioning nano-sized rechargeable battery.
Reference: Jian Yu Huang, Li Zhong, Chong Min Wang, John P. Sullivan, Wu Xu, Li Qiang Zhang, Scott X. Mao, Nicholas S. Hudak, Xiao Hua Liu, Arunkumar Subramanian, Hong You Fan, Liang Qi, Akihiro Kushima, Ju Li, In situ observation of the electrochemical lithiation of a single SnO2 nanowire electrode, Dec. 10, 2010, Science, DOI 10.1126/science.1195628.
This work was supported by EMSL and the Department of Energy Office of Science.
(*) mt.seas.upenn.edu/Stuff/JianyuHuang/Upload/S1.mov
####
About Pacific Northwest National Laboratory
Pacific Northwest National Laboratory is a Department of Energy Office of Science national laboratory where interdisciplinary teams advance science and technology and deliver solutions to America's most intractable problems in energy, the environment and national security. PNNL employs 4,900 staff, has an annual budget of nearly $1.1 billion, and has been managed by Ohio-based Battelle since the lab's inception in 1965.
For more information, please click here
Contacts:
Mary Beckman
PNNL
(509) 375-3688
Copyright © Pacific Northwest National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Appointments/Promotions/New hires/Resignations/Deaths
![]() Leibniz Prize winner Professor Dr. Oliver G. Schmidt moves to Chemnitz University of Technology: President Professor Dr. Gerd Strohmeier refers to an 'absolute top transfer' September 10th, 2021
Leibniz Prize winner Professor Dr. Oliver G. Schmidt moves to Chemnitz University of Technology: President Professor Dr. Gerd Strohmeier refers to an 'absolute top transfer' September 10th, 2021
![]() JEOL USA Welcomes New Managing Director, Hidetaka Sawada April 19th, 2021
JEOL USA Welcomes New Managing Director, Hidetaka Sawada April 19th, 2021
![]() The National Space Society Remembers Ben Bova : NSS Mourns the Loss of a Visionary NSS Leader December 2nd, 2020
The National Space Society Remembers Ben Bova : NSS Mourns the Loss of a Visionary NSS Leader December 2nd, 2020
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
Research partnerships
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() 'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








