Home > Press > ‘Instant Acid’ Method Offers New Insight into Nanoparticle Dispersal in the Environment and the Body
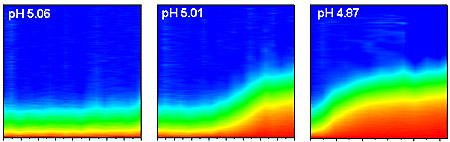 |
| Successive test runs at NIST show how clumping of typical nanoparticles in a solution depends on changes in acidity. Time after acidity jump is shown on the horizontal axis, while the vertical axis is a measure of the size of the nanoparticle aggregates. As pH goes down (and acidity up), both rate of aggregation and size of clumps of nanoparticles goes up. Credit: R. Murphy/NIST |
Abstract:
Using a chemical trick that allows them to change the acidity of a solution almost instantly, a team at the National Institute of Standards and Technology (NIST) has demonstrated a simple and effective technique for quantifying how the stability of nanoparticle solutions change when the acidity of their environment suddenly changes*. The measurement method and the problem studied are part of a broader effort at NIST to understand the environmental, health and safety implications of nanoparticles.
‘Instant Acid’ Method Offers New Insight into Nanoparticle Dispersal in the Environment and the Body
Washington, DC | Posted on June 10th, 2010Any change in nanoparticle solubility with local acidity (pH**) ultimately affects how they are distributed in the environment as well as their potential for uptake into organisms. This is crucial when designing nanoparticles for use in medicine, explains NIST chemical engineer Vivek Prabhu. "Cells in the body are very compartmentalized. There are places within the cell that have vastly different pH. For instance, in the sea of the cell, the cytosol, pH is regulated to be about 7.2, which is slightly basic. But within the lysosome, which is where things go to get broken down, the pH is about 4.5, so it's very acidic."
Nanoparticles designed for use in drug therapy or as contrast agents for medical imaging typically are coated with molecules to prevent the particles from clumping together, which would reduce their effectiveness. But the efficacy of the anti-clumping coating often depends on the pH of the environment. According to the NIST team, while it's relatively easy to put nanoparticles in a solution at a particular pH and to study the stability of the suspension over long times, it is difficult to tell what happens when the particles are suddenly exposed to a different level of acidity as often occurs in environmental and application contexts. How long does it take them to react to this change and how?
"Our idea borrows some of the materials used in photolithography to make microcircuits," says Prabhu. "There are molecules that become acids when you shine a light on them—photo acid generators. So instead of manually pouring acid into a solution and stirring it around, you start with a solution in which these molecules already are mixed and dissolved. Once you shine light on it …bam! Photolysis occurs and it becomes acidic." The acidity of the solution can be made to jump a major step—an amount chosen by the experimenter—without needing to wait for mixing or disturbing the solution. "It gives you a way to probe the nanoparticle solution dynamics at much shorter timescales than before," says Prabhu.
Using their "instant acid" technique and light scattering instruments to monitor the aggregation of nanoparticles, the NIST team followed the growth of clusters of chemically stabilized latex nanoparticles for the first few seconds after inducing the pH transition with light. Their results demonstrate that under certain conditions, the stability of the nanoparticles—their tendency to resist clumping—becomes very sensitive to pH. Studies such as these could provide a stronger foundation to design nanoparticles for applications such as targeting tumor cells that have levels of acidity markedly different from normal cells.
The work was supported in part by the National Research Council-NIST Postdoctoral Fellowship Program.
* R.J. Murphy, D. Pristinski, K. Migler, J.F. Douglas and V.M. Prabhu. Dynamic light scattering investigations of nanoparticle aggregation following a light-induced pH jump. Journal of Chemical Physics. 132, 194903 (2010) doi:10.1063/1.3425883.
** pH is the common measure used by chemists of how acidic or basic a solution is. The scale runs from 0 to 14; lower values are more acidic, higher values more basic; 7 is considered neutral.
####
For more information, please click here
Contacts:
Media Contact:
Michael Baum
(301) 975-2763
Copyright © NIST
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Academic/Education
![]() Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
![]() Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Environment
![]() Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Safety-Nanoparticles/Risk management
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
![]() Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








