Home > Press > Brown Chemists Report Promising Advance in Fuel-Cell Technology
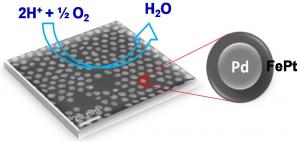 |
| Less platinum, better efficiency The multimetallic nanoparticle created by Brown University chemists for fuel-cell reactions uses a palladium core and an iron-platinum shell. Credit: Sun Lab/Brown University |
Abstract:
Chemists at Brown University have come up with a promising advance in fuel-cell technology. The team has demonstrated that a nanoparticle with a palladium core and an iron-platinum shell outperforms commercially available pure-platinum catalysts and lasts longer. The finding, reported in the Journal of the American Chemical Society, could move fuel cells a step closer to reality.
Brown Chemists Report Promising Advance in Fuel-Cell Technology
Providence, RI | Posted on May 25th, 2010Creating catalysts that can operate efficiently and last a long time is a big barrier to taking fuel-cell technology from the lab bench to the assembly line. The precious metal platinum has been the choice for many researchers, but platinum has two major downsides: It is expensive, and it breaks down over time in fuel-cell reactions.
In a new study, chemists at Brown University report a promising advance. They have created a unique core and shell nanoparticle that uses far less platinum yet performs more efficiently and lasts longer than commercially available pure-platinum catalysts at the cathode end of fuel-cell reactions.
The chemistry known as oxygen reduction reaction takes place at the fuel cell's cathode, creating water as its only waste, rather than the global-warming carbon dioxide produced by internal combustion systems. The cathode is also where up to 40 percent of a fuel cell's efficiency is lost, so "this is a crucial step in making fuel cells a more competitive technology with internal combustion engines and batteries," said Shouheng Sun, professor of chemistry at Brown and co-author of the paper in the Journal of the American Chemical Society.
The research team, which includes Brown graduate student and co-author Vismadeb Mazumder and researchers from Oak Ridge National Laboratory in Tennessee, created a five-nanometer palladium (Pd) core and encircled it with a shell consisting of iron and platinum (FePt). The trick, Mazumder said, was in molding a shell that would retain its shape and require the smallest amount of platinum to pull off an efficient reaction. The team created the iron-platinum shell by decomposing iron pentacarbonyl [Fe(CO)5] and reducing platinum acetylacetonate [Pt(acac)2], a technique Sun first reported in a 2000 Science paper. The result was a shell that uses only 30 percent platinum, although the researchers say they expect they will be able to make thinner shells and use even less platinum.
"If we don't use iron pentacarbonyl, then the platinum doesn't form on the (palladium) core," Mazumder said.
The researchers demonstrated for the first time that they could consistently produce the unique core-shell structures. In laboratory tests, the palladium/iron-platinum nanoparticles generated 12 times more current than commercially available pure-platinum catalysts at the same catalyst weight. The output also remained consistent over 10,000 cycles, at least ten times longer than commercially available platinum models that begin to deteriorate after 1,000 cycles.
The team created iron-platinum shells that varied in width from one to three nanometers. In lab tests, the group found the one-nanometer shells performed best.
"This is a very good demonstration that catalysts with a core and a shell can be made readily in half-gram quantities in the lab, they're active, and they last," Mazumder said. "The next step is to scale them up for commercial use, and we are confident we'll be able to do that."
Mazumder and Sun are studying why the palladium core increases the catalytic abilities of iron platinum, although they think it has something to do with the transfer of electrons between the core and shell metals. To that end, they are trying to use a chemically more active metal than palladium as the core to confirm the transfer of electrons in the core-shell arrangement and its importance to the catalyst's function.
Miaofang Chi and Karren More at the Oak Ridge Laboratory also contributed to the paper. The U.S. Department of Energy's Office of Energy Efficiency and Renewable Energy funded the research as part of its Fuel Cell Technologies Program.
####
For more information, please click here
Contacts:
Richard Lewis
(401) 863-3766
Copyright © Brown University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Academic/Education
![]() Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
![]() Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Materials/Metamaterials/Magnetoresistance
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








