Home > Press > Viruses harnessed to split water
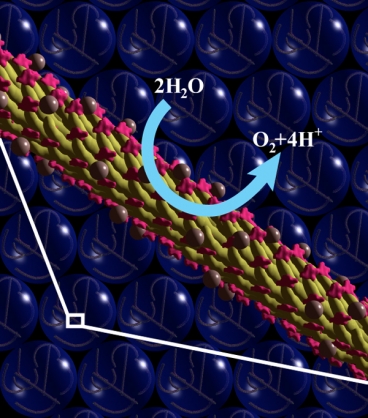 |
| A computer visualization of the biologically-based system shows the virus itself (in yellow) with molecules of pigment (in pink) and of the metal catalyst (brown spheres) attached to its surface. The pigment and catalyst cause water molecules to split apart when they come in contact. Graphic courtesy of Angela Belcher |
Abstract:
MIT team's biologically based system taps the power of sunlight directly, with the aim of turning water into hydrogen fuel.
By David L. Chandler, MIT News Office
Viruses harnessed to split water
Cambridge, MA | Posted on April 13th, 2010A team of MIT researchers has found a novel way to mimic the process by which plants use the power of sunlight to split water and make chemical fuel to power their growth. In this case, the team used a modified virus as a kind of biological scaffold that can assemble the nanoscale components needed to split the hydrogen and oxygen atoms of a water molecule.
Splitting water is one way to solve the basic problem of solar energy: It's only available when the sun shines. By using sunlight to make hydrogen from water, the hydrogen can then be stored and used at any time to generate electricity using a fuel cell, or to make liquid fuels (or be used directly) for cars and trucks.
Other researchers have made systems that use electricity, which can be provided by solar panels, to split water molecules, but the new biologically based system skips the intermediate steps and uses sunlight to power the reaction directly. The advance is described in a paper published on April 11 in Nature Nanotechnology. The Italian energy company Eni supported the research through the MIT Energy Initiative (MITEI).
The team, led by Angela Belcher, the Germeshausen Professor of Materials Science and Engineering and Biological Engineering, engineered a common, harmless bacterial virus called M13 so that it would attract and bind with molecules of a catalyst (the team used iridium oxide) and a biological pigment (zinc porphyrins). The viruses became wire-like devices that could very efficiently split the oxygen from water molecules.
Over time, however, the virus-wires would clump together and lose their effectiveness, so the researchers added an extra step: encapsulating them in a microgel matrix, so they maintained their uniform arrangement and kept their stability and efficiency.
While hydrogen obtained from water is the gas that would be used as a fuel, the splitting of oxygen from water is the more technically challenging "half-reaction" in the process, Belcher explains, so her team focused on this part. Plants and cyanobacteria (also called blue-green algae), she says, "have evolved highly organized photosynthetic systems for the efficient oxidation of water." Other researchers have tried to use the photosynthetic parts of plants directly for harnessing sunlight, but these materials can have structural stability issues.
Belcher decided that instead of borrowing plants' components, she would borrow their methods. In plant cells, natural pigments are used to absorb sunlight, while catalysts then promote the water-splitting reaction. That's the process Belcher and her team, including doctoral student Yoon Sung Nam, the lead author of the new paper, decided to imitate.
In the team's system, the viruses simply act as a kind of scaffolding, causing the pigments and catalysts to line up with the right kind of spacing to trigger the water-splitting reaction. The role of the pigments is "to act as an antenna to capture the light," Belcher explains, "and then transfer the energy down the length of the virus, like a wire. The virus is a very efficient harvester of light, with these porphyrins attached.
"We use components people have used before," she adds, "but we use biology to organize them for us, so you get better efficiency."
Using the virus to make the system assemble itself improves the efficiency of the oxygen production fourfold, Nam says. The researchers hope to find a similar biologically based system to perform the other half of the process, the production of hydrogen. Currently, the hydrogen atoms from the water get split into their component protons and electrons; a second part of the system, now being developed, would combine these back into hydrogen atoms and molecules. The team is also working to find a more commonplace, less-expensive material for the catalyst, to replace the relatively rare and costly iridium used in this proof-of-concept study.
Thomas Mallouk, the DuPont Professor of Materials Chemistry and Physics at Pennsylvania State University, who was not involved in this work, says, "This is an extremely clever piece of work that addresses one of the most difficult problems in artificial photosynthesis, namely, the nanoscale organization of the components in order to control electron transfer rates."
He adds: "There is a daunting combination of problems to be solved before this or any other artificial photosynthetic system could actually be useful for energy conversion." To be cost-competitive with other approaches to solar power, he says, the system would need to be at least 10 times more efficient than natural photosynthesis, be able to repeat the reaction a billion times, and use less expensive materials. "This is unlikely to happen in the near future," he says. "Nevertheless, the design idea illustrated in this paper could ultimately help with an important piece of the puzzle."
Belcher will not even speculate about how long it might take to develop this into a commercial product, but she says that within two years she expects to have a prototype device that can carry out the whole process of splitting water into oxygen and hydrogen, using a self-sustaining and durable system.
####
For more information, please click here
Copyright © MIT
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Academic/Education
![]() Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
![]() Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Grants/Sponsored Research/Awards/Scholarships/Gifts/Contests/Honors/Records
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Solar/Photovoltaic
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() Charged “molecular beasts” the basis for new compounds: Researchers at Leipzig University use “aggressive” fragments of molecular ions for chemical synthesis November 3rd, 2023
Charged “molecular beasts” the basis for new compounds: Researchers at Leipzig University use “aggressive” fragments of molecular ions for chemical synthesis November 3rd, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








