Home > Press > Layered Graphene Sheets Could Solve Hydrogen Storage Issues
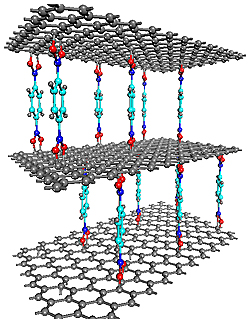 |
| A graphene-oxide framework (GOF), formed of layers of graphene connected by boron-carboxylic “pillars.” GOFs such as this one are just beginning to be explored as a potential storage medium for hydrogen and other gases. Credit: NIST |
Abstract:
Graphene—carbon formed into sheets a single atom thick—now appears to be a promising base material for capturing hydrogen, according to recent research* at the National Institute of Standards and Technology (NIST) and the University of Pennsylvania. The findings suggest stacks of graphene layers could potentially store hydrogen safely for use in fuel cells and other applications.
Layered Graphene Sheets Could Solve Hydrogen Storage Issues
Gaithersburg, MD | Posted on March 19th, 2010Graphene has become something of a celebrity material in recent years due to its conductive, thermal and optical properties, which could make it useful in a range of sensors and semiconductor devices. The material does not store hydrogen well in its original form, according to a team of scientists studying it at the NIST Center for Neutron Research. But if oxidized graphene sheets are stacked atop one another like the decks of a multilevel parking lot, connected by molecules that both link the layers to one another and maintain space between them, the resulting graphene-oxide framework (GOF) can accumulate hydrogen in greater quantities.
Inspired to create GOFs by the metal-organic frameworks that are also under scrutiny for hydrogen storage, the team is just beginning to uncover the new structures' properties. "No one else has ever made GOFs, to the best of our knowledge," says NIST theorist Taner Yildirim. "What we have found so far, though, indicates GOFs can hold at least a hundred times more hydrogen molecules than ordinary graphene oxide does. The easy synthesis, low cost and non-toxicity of graphene make this material a promising candidate for gas storage applications."
The GOFs can retain 1 percent of their weight in hydrogen at a temperature of 77 degrees Kelvin and ordinary atmospheric pressure—roughly comparable to the 1.2 percent that some well-studied metal-organic frameworks can hold, Yildirim says.
Another of the team's potentially useful discoveries is the unusual relationship that GOFs exhibit between temperature and hydrogen absorption. In most storage materials, the lower the temperature, the more hydrogen uptake normally occurs. However, the team discovered that GOFs behave quite differently. Although a GOF can absorb hydrogen, it does not take in significant amounts at below 50 Kelvin (-223 degrees Celsius). Moreover, it does not release any hydrogen below this "blocking temperature"—suggesting that, with further research, GOFs might be used both to store hydrogen and to release it when it is needed, a fundamental requirement in fuel cell applications.
Some of the GOFs' capabilities are due to the linking molecules themselves. The molecules the team used are all benzene-boronic acids that interact strongly with hydrogen in their own right. But by keeping several angstroms of space between the graphene layers—akin to the way pillars hold up a ceiling—they also increase the available surface area of each layer, giving it more spots for the hydrogen to latch on.
According to the team, GOFs will likely perform even better once the team explores their parameters in more detail. "We are going to try to optimize the performance of the GOFs and explore other linking molecules as well," says Jacob Burress, also of NIST. "We want to explore the unusual temperature dependence of absorption kinetics, as well as whether they might be useful for capturing greenhouse gases such as carbon dioxide and toxins like ammonia."
The research is funded in part by the Department of Energy.
* J. Burress, J. Simmons, J. Ford and T.Yildirim. "Gas adsorption properties of graphene-oxide-frameworks and nanoporous benzene-boronic acid polymers." To be presented at the March meeting of the American Physical Society (APS) in Portland, Ore., March 18, 2010. An abstract is available at meetings.aps.org/Meeting/MAR10/Event/122133
####
About NIST
From automated teller machines and atomic clocks to mammograms and semiconductors, innumerable products and services rely in some way on technology, measurement, and standards provided by the National Institute of Standards and Technology.
Founded in 1901, NIST is a non-regulatory federal agency within the U.S. Department of Commerce. NIST's mission is to promote U.S. innovation and industrial competitiveness by advancing measurement science, standards, and technology in ways that enhance economic security and improve our quality of life.
For more information, please click here
Contacts:
Media Contact
Chad Boutin
(301) 975-4261
Copyright © NIST
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Materials/Metamaterials/Magnetoresistance
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Automotive/Transportation
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








