 |
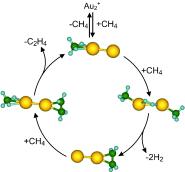
| Au pairs: Whereas one methane molecule is not dehydrogenated by free gold-dimer cations, the cooperative interaction of two methane ligands initiates a catalytic formation of ethylene. The cycle is closed by the adsorption of a third methane molecule that cooperatively activates the release of ethylene (see scheme yellow Au, green C, blue H). This cycle is derived from ab initio calculations and gas-phase reaction kinetics measurements. |
Abstract:
Catalytic dimers of gold atoms make ethylene from methane
Golden Pairs
Weinheim, Germany | Posted on January 19th, 2010Ethylene (ethene, CH2=CH2) is a primary feedstock for chemical industry, and particularly for the production of plastics like polyethylene and polystyrene. Ethylene is currently made by the steam cracking of fossil fuel fractions. A possible alternative to this may be the production of ethylene from methane (CH4), because although fossil fuel supplies are slowly declining, methane is still found in giant natural gas deposits. The problem is that the carbon-hydrogen bonds in methane are very difficult to break. It thus usually takes extreme conditions to induce the carbon in methane to form bonds with other carbon atoms. Furthermore, this reaction usually produces a mixture. Scientists working with Thorsten M. Bernhardt at the University of Ulm (Germany) and Uzi Landman at the Georgia Institute of Technology (Atlanta, USA), have now found a process by which methane can be selectively converted into ethylene at low pressures and temperatures. Free gold dimers catalyze the reaction, the researchers report in the journal Angewandte Chemie.
"Methane activation, meaning the ‘cracking' of C-H bonds, is a very complex process," explain the scientists, "which must be understood at the molecular level before practically applicable catalytic processes can be developed." To investigate this, the team carried out experiments with different catalytic metal clusters (aggregates of a few metal atoms) as model systems. In tests with particles made of a few gold atoms, they found that positively charged particles made of two gold atoms (Au2+) selectively convert methane into ethylene in the gas phase.
Through experiments in which intermediates of the reaction were "trapped", as well as model computations, the researchers were able to formulate a reaction mechanism for this catalytic cycle. Each gold atom of the gold dimers binds to a methane molecule; hydrogen is split off and the two carbon atoms form a single bond to each other. This ethylene precursor initially remains bound to one of the gold atoms, and the freed gold atom binds to a new methane molecule. In the last step, another methane molecule displaces the ethylene precursor from its spot on the gold atom and ethylene is released. At this point the reaction cycle can begin again.
"Both the activation of the carbon-hydrogen bonds of the methane and the subsequent splitting off of the ethylene molecule require cooperative action of several atoms bound to the gold dimer," Berhnardt and Landman explain further details of the mechanism. "Our insights are not only of fundamental interest, but may also be of practical use."
Author: Thorsten M. Bernhardt, Universität Ulm (Germany), www.uni-ulm.de/iok/bernhardt/
Title: Methane Activation and Catalytic Ethylene Formation on Free Au2+ Ions
Angewandte Chemie International Edition 2010, 49, No. 5, 980-983,
Permalink: dx.doi.org/10.1002/anie.200905643
####
About Wiley InterScience
Wiley InterScience (www.interscience.wiley.com) provides access to over 3 million articles across nearly 1500 journals and 7000 Online Books and major reference works. It also holds industry leading databases such as The Cochrane Library, chemistry databases and the acclaimed Current Protocols laboratory manuals.
Wiley InterScience is one of the world's premiere resources for study, teaching and advanced research.
For more information, please click here
Contacts:
Editorial office
Amy Molnar (US)
Jennifer Beal (UK)
Alina Boey (Asia)
Copyright © Wiley InterScience
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Industrial
![]() Boron nitride nanotube fibers get real: Rice lab creates first heat-tolerant, stable fibers from wet-spinning process June 24th, 2022
Boron nitride nanotube fibers get real: Rice lab creates first heat-tolerant, stable fibers from wet-spinning process June 24th, 2022
![]() Nanotubes: a promising solution for advanced rubber cables with 60% less conductive filler June 1st, 2022
Nanotubes: a promising solution for advanced rubber cables with 60% less conductive filler June 1st, 2022
![]() Protective equipment with graphene nanotubes meets the strictest ESD safety standards March 25th, 2022
Protective equipment with graphene nanotubes meets the strictest ESD safety standards March 25th, 2022
![]() OCSiAl receives the green light for Luxembourg graphene nanotube facility project to power the next generation of electric vehicles in Europe March 4th, 2022
OCSiAl receives the green light for Luxembourg graphene nanotube facility project to power the next generation of electric vehicles in Europe March 4th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








