Home > Press > New superconductivity mechanism found in iron compound
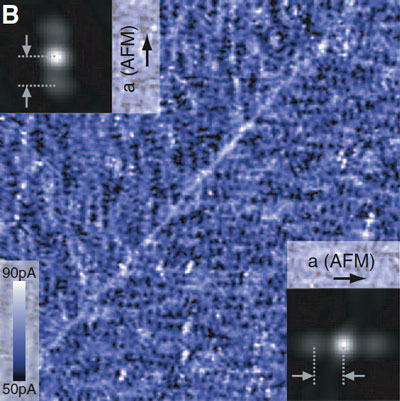 |
| STM scan showing a 96-nanometer square of an iron-based superconductor shows electrons lined up in parallel rows suggesting a 'liquid-crystal' state of the electron fluid. The parallel arrangements appear in random domains across the entire crystal, oriented either vertically or horizontally. The diagonal line across this image is the boundary between two domains. The discovery of this arrangement indicates that the mechanism of iron-based superconductors is more complex than previously believed, and may be similar to the mechanism in cuprates. |
Abstract:
A surprising discovery by Cornell researchers of electronic liquid crystal states in an iron-based, high-temperature superconductor is another step toward understanding superconductivity and using it in such applications as power transmission.
New superconductivity mechanism found in iron compound
Ithaca, NY | Posted on January 8th, 2010"Because these findings appear similar to what we have observed in the parent state of [copper-based] superconductors, it suggests this could represent a common factor in the mechanism for high-temperature superconductivity in these two otherwise very different families of materials," said team leader J.C. Séamus Davis, Cornell's J.D. White Distinguished Professor of Physical Sciences and director of the U.S. Department of Energy's Center for Emergent Superconductivity. The researchers describe their findings in the Jan. 8 issue of the journal Science.
Many theorists had expected the iron-based materials to act more like conventional metal superconductors, where electrons pair up to carry current effortlessly but without requiring any specific spatial arrangements of the atoms in the metal. These materials conduct electricity with zero resistance only at temperatures near absolute zero, or -270 degrees Celsius (-454 Fahrenheit).
Cuprate, or copper-based, and newly discovered iron-based superconductors operate at a range of warmer, though still chilly, temperatures (up to -120 degrees Celsius or -184 Fahrenheit for cuprates and -220 degrees Celsius or -364 Fahrenheit for iron-based compounds) that make them potentially more practical for such large-scale, real-world applications as zero-loss power transmission lines.
Cuprates are oxides of copper "doped" with various other atoms. Iron-based superconductors -- first demonstrated only in 2008 -- are mostly doped compounds of iron and arsenic. Somehow the doping distorts the crystal structure of the material in a way that makes it possible for electrons to flow without resistance. Understanding how this works could open the door to engineering even higher-temperature, or ideally, room-temperature, versions.
The scientists used a specially built scanning tunneling microscope (STM) in Davis' lab at Cornell, in which a tiny probe is moved across a surface in steps smaller than the width of an atom. By varying a current flowing between the probe and the surface, Davis is able to read out a spectrum of the energy levels of electrons in the material and produce a picture of the distribution of the electrons. Davis was recently awarded the Kamerlingh-Onnes Prize for inventing this technique.
Davis and colleagues examined "underdoped" samples of a compound of calcium, iron, cobalt and arsenic that becomes a superconductor when the amount of cobalt doping is increased. The particular material they used, made by Paul Canfield at the U.S. Department of Energy's (DOE) Ames Laboratory in Iowa, was a crucial choice, Davis said, because it could be sliced to produce an atomically flat and perfectly debris-free surface needed for the STM techniques.
It became clear to the team that they were on to something very different than expected. They observed static, nanoscale lineups of electrons spanning about eight times the distance between individual iron atoms, all aligned along one axis of the underlying crystal, reminiscent of the way molecules line up in a liquid crystal.
Liquid crystals, used in electronic displays, are a sort of intermediate state between liquid and solid in which molecules line up in parallel rows that can control the passage of light. In the solid crystals of materials like high-temperature superconductors, electrons do not remain attached to individual atoms but behave like a fluid, and here, Davis said, the electrons seem to be in a state analogous to a liquid crystal. "You can't use ordinary solid-state physics to understand materials this complicated," he said.
The scientists also found that the electrons that are free to travel through the material do so in a direction perpendicular to these aligned electronic liquid crystal states. This indicates that the electrons carrying the current are distinct from those apparently aligned in the electronic liquid crystals.
The next step will be to see how these conditions affect the superconductivity of the material when it is transformed to a superconductor.
The observations are "amazingly similar" to what Davis and his team have seen in cuprates. "If we're able to relate our observations in the iron-based superconductors to what happens in cuprate superconductors, it may help us understand the overall mechanism for high-temperature superconductivity in all of these materials. That understanding could, in turn, help us to engineer new materials with improved superconducting properties for energy applications," Davis said.
Scientists from the National High Magnetic Field Laboratory at Florida State University and St. Andrews University, Scotland, collaborated on this research, funded by DOE's Office of Science; the National Science Foundation; the Office of Naval Research; the U.K. Engineering and Physical Sciences Research Council; and the Scottish Funding Council.
Images and supplementary materials on the research are available on Davis' Web site at people.ccmr.cornell.edu/~jcdavis/
####
About Cornell University
Once called "the first American university" by educational historian Frederick Rudolph, Cornell University represents a distinctive mix of eminent scholarship and democratic ideals. Adding practical subjects to the classics and admitting qualified students regardless of nationality, race, social circumstance, gender, or religion was quite a departure when Cornell was founded in 1865.
Today's Cornell reflects this heritage of egalitarian excellence. It is home to the nation's first colleges devoted to hotel administration, industrial and labor relations, and veterinary medicine. Both a private university and the land-grant institution of New York State, Cornell University is the most educationally diverse member of the Ivy League.
For more information, please click here
Contacts:
Media Contact:
Blaine Friedlander
(607) 254-8093
Cornell Chronicle:
Anne Ju
(607) 255-9735
Copyright © Cornell University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Materials/Metamaterials/Magnetoresistance
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Tools
![]() Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








